 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Solian, Barhemsys, others |
| Other names | Aminosultopride; AST; APD-421; APD421; APD-403; APD403; DAN-2163; DAN2163 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth, intravenous |
| Drug class | Dopamine D2 and D3 receptor antagonist; Serotonin 5-HT2B and 5-HT7 receptor antagonist; Antipsychotic; Antidepressant; Antiemetic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 48%[5][6] |
| Protein binding | 16%[6] |
| Metabolism | Liver (minimal; most excreted unchanged)[6] |
| Elimination half-life | 12 hours[5] |
| Excretion | Kidney[5] (23–46%),[7][8] Faecal[6] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.068.916 |
| Chemical and physical data | |
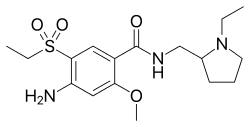
| Formula | C17H27N3O4S |
| Molar mass | 369.48 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Amisulpride, sold under the brand names Solian and Barhemsys, is a medication used in the treatment of schizophrenia, acute psychotic episodes, depression, and nausea and vomiting.[9][6] It is specifically used at lower doses intravenously to prevent and treat postoperative nausea and vomiting;[9] at low doses by mouth to treat depression; and at higher doses by mouth to treat psychosis.[6][10][11]
It is usually classed with the atypical antipsychotics. Chemically it is a benzamide and like other benzamide antipsychotics, such as sulpiride, it is associated with a high risk of elevating blood levels of the lactation hormone, prolactin (thereby potentially causing the absence of the menstrual cycle, breast enlargement, even in males, breast milk secretion not related to breastfeeding, impaired fertility, impotence, breast pain, etc.), and a low risk, relative to the typical antipsychotics, of causing movement disorders.[12][13][14]
Amisulpride is indicated for use in the United States in adults for the prevention of postoperative nausea and vomiting (PONV), either alone or in combination with an antiemetic of a different class; and to treat PONV in those who have received antiemetic prophylaxis with an agent of a different class or have not received prophylaxis.[9]
Amisulpride is believed to work by blocking, or antagonizing, the dopamine D2 receptor, reducing its signalling. The effectiveness of amisulpride in treating dysthymia and the negative symptoms of schizophrenia is believed to stem from its blockade of the presynaptic dopamine D2 and D3 autoreceptors. These presynaptic receptors regulate the release of dopamine into the synapse, so by blocking them amisulpride increases dopamine concentrations in the synapse. This increased dopamine concentration is theorized to act on dopamine D1 receptors to relieve depressive symptoms (in dysthymia) and the negative symptoms of schizophrenia.[11]
It was introduced by Sanofi-Aventis in the 1990s. Its patent expired by 2008, and generic formulations became available.[15] It is marketed in all English-speaking countries except for Canada.[14]
- ^ "Australian Product Information – Solian (Amisulpride) Tablets And Solution". TGA eBS. Retrieved 10 May 2020.
- ^ a b "Amisulpride (Barhemsys) Use During Pregnancy". Drugs.com. 2 September 2020. Retrieved 24 September 2020.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 16 August 2023.
- ^ "Amisulpride 100 mg Tablets - Summary of Product Characteristics (SmPC)". (emc). 5 July 2019. Archived from the original on 26 February 2020. Retrieved 26 February 2020.
- ^ a b c Rosenzweig P, Canal M, Patat A, Bergougnan L, Zieleniuk I, Bianchetti G (January 2002). "A review of the pharmacokinetics, tolerability and pharmacodynamics of amisulpride in healthy volunteers". Human Psychopharmacology. 17 (1): 1–13. doi:10.1002/hup.320. PMID 12404702. S2CID 23877366.
- ^ a b c d e f "Solian tablets and solution product information" (PDF). TGA eBusiness Services. Sanofi-Aventis Australia Pty Ltd. 27 September 2019. Retrieved 26 February 2020.
- ^ Caccia S (May 2000). "Biotransformation of post-clozapine antipsychotics: pharmacological implications". Clinical Pharmacokinetics. 38 (5): 393–414. doi:10.2165/00003088-200038050-00002. PMID 10843459. S2CID 68853079.
- ^ Noble S, Benfield P (December 1999). "Amisulpride: A Review of its Clinical Potential in Dysthymia". CNS Drugs. 12 (6): 471–483. doi:10.2165/00023210-199912060-00005. S2CID 71691764.
- ^ a b c "Barhemsys (amisulpride) injection, for intravenous use" (PDF). U.S. Food and Drug Administration (FDA). February 2020. Retrieved 26 February 2020.
- ^ "Amisulpride". AdisInsight. 24 October 2021. Retrieved 24 October 2024.
- ^ a b Cite error: The named reference
PaniGessa2002was invoked but never defined (see the help page). - ^ Cite error: The named reference
AMHwas invoked but never defined (see the help page). - ^ Cite error: The named reference
Lancetwas invoked but never defined (see the help page). - ^ a b Brayfield A, ed. (June 2017). "Amisulpride: Martindale: The Complete Drug Reference". MedicineComplete. Pharmaceutical Press. Retrieved 5 August 2017.
- ^ De Silva V, Hanwella R (April 2008). "Pharmaceutical patents and the quality of mental healthcare in low- and middle-income countries". The Psychiatrist. 32 (4): 121–23. doi:10.1192/pb.bp.107.015651.