 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /æmˈloʊdɪˌpiːn/[1] |
| Trade names | Norvasc, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692044 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| Drug class | Calcium channel blocker |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 64–90% |
| Protein binding | 93%[7] |
| Metabolism | Liver |
| Metabolites | Various inactive pyrimidine metabolites |
| Onset of action | Highest availability 6–12 hours after oral dose[10] |
| Elimination half-life | 30–50 hours |
| Duration of action | At least 24 hours[10] |
| Excretion | Urine[10] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.102.428 |
| Chemical and physical data | |
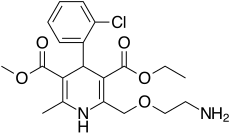
| Formula | C20H25ClN2O5 |
| Molar mass | 408.88 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Amlodipine, sold under the brand name Norvasc among others, is a calcium channel blocker medication used to treat high blood pressure, coronary artery disease (CAD)[10] and variant angina (also called Prinzmetal angina or coronary artery vasospasm, among other names).[11] It is taken orally (swallowed by mouth).[10]
Common side effects include swelling, feeling tired, abdominal pain, and nausea.[10] Serious side effects may include low blood pressure or heart attack.[10] Whether use is safe during pregnancy or breastfeeding is unclear.[2][10] When used by people with liver problems, and in elderly individuals, doses should be reduced.[10] Amlodipine works partly by vasodilation (relaxing the arteries and increasing their diameter).[10] It is a long-acting calcium channel blocker of the dihydropyridine type.[10]
Amlodipine was patented in 1982, and approved for medical use in 1990.[12] It is on the World Health Organization's List of Essential Medicines.[13] It is available as a generic medication.[10][14] In 2022, it was the fifth most commonly prescribed medication in the United States, with more than 70 million prescriptions.[15][16] In Australia, it was one of the top 10 most prescribed medications between 2017 and 2023.[17]
- ^ "Medical Definition of Amlodipine". www.merriam-webster.com. Archived from the original on 8 November 2016. Retrieved 5 July 2017.
- ^ a b "Amlodipine Use During Pregnancy". Drugs.com. 28 October 2019. Archived from the original on 28 December 2019. Retrieved 29 December 2019.
- ^ "Poisons Standard June 2017". legislation.gov.au. 29 May 2017. Archived from the original on 13 December 2020. Retrieved 7 January 2018.
- ^ "Norvasc Product and Consumer Medicine Information Licence". TGA eBS. Archived from the original on 20 June 2022. Retrieved 19 June 2022.
- ^ "Norvasc product information". Health Canada. 25 April 2012. Archived from the original on 20 June 2022. Retrieved 19 June 2022.
- ^ Cite error: The named reference
Istin SmPCwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Norvasc FDA labelwas invoked but never defined (see the help page). - ^ "Norliqva- amlodipine solution". DailyMed. 28 February 2022. Archived from the original on 20 June 2022. Retrieved 19 June 2022.
- ^ "Amlodipine" (PDF). List of nationally authorised medicinal products. European Medicines Agency. Archived (PDF) from the original on 3 March 2022. Retrieved 20 June 2022.
- ^ a b c d e f g h i j k l "Amlodipine Besylate". Drugs.com. American Society of Hospital Pharmacists. Archived from the original on 4 June 2016. Retrieved 22 July 2016.
- ^ Boden WE (2012). Goldman's Cecil Medicine (24th ed.). Saunders. ISBN 978-1-4377-1604-7. Archived from the original on 26 August 2023. Retrieved 26 August 2023.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 465. ISBN 9783527607495. Archived from the original on 27 August 2021. Retrieved 1 June 2020.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Amlodipine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ "Medicines in the health system". Australian Institute of Health and Welfare. 2 July 2024. Retrieved 30 September 2024.