| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ammonia[1]
| |||
| Systematic IUPAC name
Azane | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 3587154 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.760 | ||
| EC Number |
| ||
| 79 | |||
| KEGG | |||
| MeSH | Ammonia | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1005 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| NH3 | |||
| Molar mass | 17.031 g·mol−1 | ||
| Appearance | Colourless gas | ||
| Odor | Strong pungent odour | ||
| Density |
| ||
| Melting point | −77.73 °C (−107.91 °F; 195.42 K) (Triple point at 6.060 kPa, 195.4 K) | ||
| Boiling point | −33.34 °C (−28.01 °F; 239.81 K) | ||
| Critical point (T, P) | 132.4 °C (405.5 K), 111.3 atm (11,280 kPa) | ||
| |||
| Solubility | soluble in chloroform, ether, ethanol, methanol | ||
| Vapor pressure | 857.3 kPa | ||
| Acidity (pKa) | 32.5 (−33 °C),[6] 9.24 (of ammonium) | ||
| Basicity (pKb) | 4.75 | ||
| Conjugate acid | Ammonium | ||
| Conjugate base | Amide | ||
| −18.0×10−6 cm3/mol | |||
Refractive index (nD)
|
1.3327 | ||
| Viscosity |
| ||
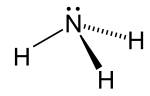
| Structure | |||
| C3v | |||
| Trigonal pyramid | |||
| 1.42 D | |||
| Thermochemistry | |||
Std molar
entropy (S⦵298) |
193 J/(mol·K)[8] | ||
Std enthalpy of
formation (ΔfH⦵298) |
−46 kJ/mol[8] | ||
| Hazards | |||
| GHS labelling:[11] | |||
  
| |||
| Danger | |||
| H314, H331, H410 | |||
| P260, P273, P280, P303+P361+P353, P304+P340+P311, P305+P351+P338+P310 | |||
| NFPA 704 (fire diamond) | |||
| 651 °C (1,204 °F; 924 K) | |||
| Explosive limits | 15.0–33.6% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
350 mg/kg (rat, oral)[9] | ||
LC50 (median concentration)
|
| ||
LCLo (lowest published)
|
| ||
| NIOSH (US health exposure limits):[12] | |||
PEL (Permissible)
|
50 ppm (25 ppm ACGIH-TLV; 35 ppm STEL) | ||
REL (Recommended)
|
TWA 25 ppm (18 mg/m3) ST 35 ppm (27 mg/m3) | ||
IDLH (Immediate danger)
|
300 ppm | ||
| Safety data sheet (SDS) | ICSC 0414 (anhydrous) | ||
| Related compounds | |||
Related nitrogen hydrides
|
|||
Related compounds
|
|||
| Supplementary data page | |||
| Ammonia (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers.[13] Around 70% of ammonia produced industrially is used to make fertilisers[14] in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil.
Ammonia, either directly or indirectly, is also a building block for the synthesis of many chemicals.
Ammonia occurs in nature and has been detected in the interstellar medium. In many countries, it is classified as an extremely hazardous substance.[15]
Ammonia is produced biologically in a process called nitrogen fixation, but even more is generated industrially by the Haber process. The process helped revolutionize agriculture by providing cheap fertilizers. The global industrial production of ammonia in 2021 was 235 million tonnes.[16][17] Industrial ammonia is transported by road in tankers, by rail in tank wagons, by sea in gas carriers, or in cylinders.[18]
Ammonia boils at −33.34 °C (−28.012 °F) at a pressure of one atmosphere, but the liquid can often be handled in the laboratory without external cooling. Household ammonia or ammonium hydroxide is a solution of ammonia in water.
- ^ "Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005" (PDF). Archived (PDF) from the original on 9 October 2022.
- ^ "Gases – Densities". The Engineering Toolbox. Retrieved 3 March 2016.
- ^ Yost, Don M. (2007). "Ammonia and Liquid Ammonia Solutions". Systematic Inorganic Chemistry. Read Books. p. 132. ISBN 978-1-4067-7302-6.
- ^ Blum, Alexander (1975). "On crystalline character of transparent solid ammonia". Radiation Effects and Defects in Solids. 24 (4): 277. Bibcode:1975RadEf..24..277B. doi:10.1080/00337577508240819.
- ^ "Ammonia". The American Chemical Society. 8 February 2021. Retrieved 20 March 2024.
- ^ Perrin, D. D. (1982). Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution (2nd ed.). Oxford: Pergamon Press.
- ^ Iwasaki, Hiroji; Takahashi, Mitsuo (1968). "Studies on the transport properties of fluids at high pressure". The Review of Physical Chemistry of Japan. 38 (1).
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles (6th ed.). Houghton Mifflin. p. A22. ISBN 978-0-618-94690-7.
- ^ "Ammonia, Anhydrous Safety Data Sheet" (PDF). University of Florida. Retrieved 19 April 2024.
- ^ a b "Ammonia". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Sigma-Aldrich Co., Ammonia.
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0028". National Institute for Occupational Safety and Health (NIOSH).
- ^ Ritchie, Hannah. "How many people does synthetic fertilizer feed?". Our World in Data. Retrieved 4 September 2021.
- ^ "Ammonia Technology Roadmap – Analysis". IEA. 11 October 2021.
- ^ "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities". United States: Government Printing Office.
- ^ "Global ammonia annual production capacity". Statistia.
- ^ "Mitsubishi Heavy Industries BrandVoice: Scaling Ammonia Production for the World's Food Supply". Forbes. 29 October 2021.
- ^ Shreve, R. Norris; Brink, Joseph (1977). Chemical Process Industries (4th ed.). McGraw-Hill. p. 276. ISBN 978-0-07-057145-7.


