| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ammonium ion
| |||
| Systematic IUPAC name
Azanium[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| MeSH | D000644 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| [NH4]+ | |||
| Molar mass | 18.039 g·mol−1 | ||
| Acidity (pKa) | 9.25 | ||
| Conjugate base | Ammonia | ||
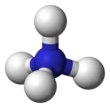
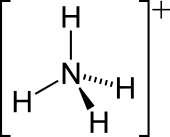
| Structure | |||
| Tetrahedral | |||
| Related compounds | |||
Other cations
|
| ||
Related compounds
|
Ammonium radical •NH4 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) molecular ion with the chemical formula NH+4 or [NH4]+. It is formed by the addition of a proton (a hydrogen nucleus) to ammonia (NH3). Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations ([NR4]+), where one or more hydrogen atoms are replaced by organic or other groups (indicated by R). Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle.[2] As such, human impact in recent years could have an effect on the biological communities that depend on it.
- ^ International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. pp. 71,105,314. Electronic version.
- ^ Schlesinger, William H.; Bernhardt, Emily S. (2020-01-01), Schlesinger, William H.; Bernhardt, Emily S. (eds.), "Chapter 12 - The Global Cycles of Nitrogen, Phosphorus and Potassium", Biogeochemistry (Fourth Edition), Academic Press, pp. 483–508, doi:10.1016/b978-0-12-814608-8.00012-8, ISBN 978-0-12-814608-8, retrieved 2024-03-08