
| |
| |

| |
| Names | |
|---|---|
| IUPAC name
Ammonium dichromate
| |
| Other names
Ammonium bichromate
Ammonium pyrochromate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.221 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1439 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| (NH4)2Cr2O7 | |
| Molar mass | 252.07 g/mol |
| Appearance | Orange-red crystals |
| Odor | odorless |
| Density | 2.115 g/cm3 |
| Melting point | 180 °C (356 °F; 453 K) decomposes |
| 18.2 g/100 ml (0 °C) 35.6 g/100 ml (20 °C) 40 g/100 ml (25 °C) 156 g/100 ml (100 °C) | |
| Solubility | insoluble in acetone soluble in ethanol |
| Structure | |
| monoclinic | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Very toxic, explosive, oxidizing, carcinogenic, mutagenic, dangerous for the environment |
| GHS labelling: | |
      [1] [1]
| |
| H272, H301, H312, H314, H317, H330, H334, H340, H350, H360, H372, H410[1] | |
| P201, P220, P260, P273, P280, P284[1] | |
| NFPA 704 (fire diamond) | |
| 190 °C (374 °F; 463 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
20–250 mg/kg |
| Safety data sheet (SDS) | ICSC 1368 |
| Related compounds | |
Other cations
|
Potassium dichromate Sodium dichromate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
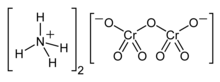
Ammonium dichromate is an inorganic compound with the formula (NH4)2Cr2O7. In this compound, as in all chromates and dichromates, chromium is in a +6 oxidation state, commonly known as hexavalent chromium. It is a salt consisting of ammonium ions and dichromate ions.
Ammonium dichromate is sometimes known as Vesuvian Fire, because of its use in demonstrations of tabletop "volcanoes".[2] However, this demonstration has become unpopular in schools due to the compound's carcinogenic nature. It has also been used in pyrotechnics and in the early days of photography.
- ^ a b c Sigma-Aldrich Co., Ammonium dichromate. Retrieved on 2013-07-20.
- ^ "Ammonium Dichromate Volcano". Chemistry Comes Alive!. J. Chem. Educ. (dead link 29 March 2021)
