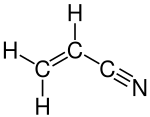
In organic chemistry, ammoxidation is a process for the production of nitriles (R−C≡N) using ammonia (NH3) and oxygen (O2). It is sometimes called the SOHIO process, acknowledging that ammoxidation was developed at Standard Oil of Ohio.[1][2] The usual substrates are alkenes. Several million tons of acrylonitrile are produced in this way annually:[3][4]
- ^ Pollak, Peter; Romeder, Gérard; Hagedorn, Ferdinand; Gelbke, Heinz-Peter (2000). "Nitriles". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_363. ISBN 3527306730.
- ^ "Sohio Acrylonitrile Process - American Chemical Society". American Chemical Society. Retrieved 11 July 2017.
- ^ Peter Pollak, Gérard Romeder, Ferdinand Hagedorn, Heinz-Peter Gelbke "Nitriles" Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_363
- ^ "The Sohio Acrylonitrile Process". National Historic Chemical Landmarks. American Chemical Society. Archived from the original on February 23, 2013. Retrieved March 25, 2013.
