 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| License data |
|
| Routes of administration | Oral, IM, IV, Rectal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 8–42 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.300 |
| Chemical and physical data | |
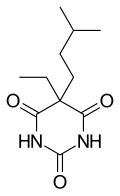
| Formula | C11H18N2O3 |
| Molar mass | 226.276 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Amobarbital (formerly known as amylobarbitone or sodium amytal as the soluble sodium salt) is a drug that is a barbiturate derivative. It has sedative-hypnotic properties. It is a white crystalline powder with no odor and a slightly bitter taste. It was first synthesized in Germany in 1923. It is considered a short to intermediate acting barbiturate.[citation needed]
If amobarbital is taken for extended periods of time, physiological and psychological dependence can develop. Amobarbital withdrawal mimics delirium tremens and may be life-threatening. Amobarbital was manufactured by Eli Lilly and Company in the US under the brand name Amytal in bright blue bullet shaped capsules (known as Pulvules) or pink tablets (known as Diskets)[2] containing 50, 100, or 200 milligrams of the drug. The drug was also manufactured generically.
Amobarbital was widely misused, known as "Blue Heavens" on the street. Amytal, as well as Tuinal, a combination drug containing equal quantities of secobarbital and amobarbital, were both manufactured by Eli Lilly until the late 1990s. However, as the popularity of benzodiazepines increased, prescriptions for these medications became increasingly rare beginning in the mid to late 1980s.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Winek, Charles L. (1965-02-01). "Dosage Form Names and Product Identification". American Journal of Health-System Pharmacy. 22 (2): 84. doi:10.1093/ajhp/22.2.82. ISSN 1079-2082.