 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Inocor |
| Other names | inamrinone (USAN US) |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | 10 to 49% |
| Metabolism | Hepatic |
| Elimination half-life | 5 to 8 hours |
| Excretion | Renal (63%) and fecal (18%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.056.700 |
| Chemical and physical data | |
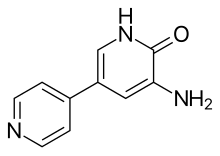
| Formula | C10H9N3O |
| Molar mass | 187.202 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Amrinone, also known as inamrinone, and sold as Inocor, is a pyridine phosphodiesterase 3 inhibitor.[1] It is a drug that may improve the prognosis in patients with congestive heart failure.[2] Amrinone has been shown to increase the contractions initiated in the heart by high-gain calcium induced calcium release (CICR).[3] The positive inotropic effect of amrinone is mediated by the selective enhancement of high-gain CICR, which contributes to the contraction of myocytes by phosphorylation through cAMP dependent protein kinase A (PKA) and Ca2+ calmodulin kinase pathways.[3]
- ^ Hamada Y, Kawachi K, Yamamoto T, Nakata T, Kashu Y, Sato M, Watanabe Y (August 1999). "Effects of single administration of a phosphodiesterase III inhibitor during cardiopulmonary bypass: comparison of milrinone and amrinone". Japanese Circulation Journal. 63 (8): 605–609. doi:10.1253/jcj.63.605. PMID 10478810.
- ^ Cite error: The named reference
AJC.1981.48was invoked but never defined (see the help page). - ^ a b Xiong W, Ferrier GR, Howlett SE (August 2004). "Diminished inotropic response to amrinone in ventricular myocytes from myopathic hamsters is linked to depression of high-gain Ca2+-induced Ca2+ release". The Journal of Pharmacology and Experimental Therapeutics. 310 (2): 761–773. doi:10.1124/jpet.103.064873. PMID 15064331. S2CID 6036283.