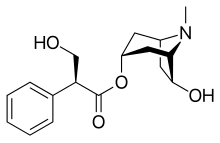
 | |
| Clinical data | |
|---|---|
| Other names | 7β-hydroxyhyoscyamine |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.164.962 |
| Chemical and physical data | |
| Formula | C17H23NO4 |
| Molar mass | 305.374 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Anisodamine, also known as 7β-hydroxyhyoscyamine, is an anticholinergic and α1 adrenergic receptor antagonist used in the treatment of acute circulatory shock in China.[1] It is given orally or by injection, as a racemic mixture (racanisodamine) or as a hydrobromide salt.[2] Eye drops at 0.5% concentration for slowing the progression of myopia is also available in China.[3]
Anisodamine is a naturally occurring tropane alkaloid found in some plants of the family Solanaceae including Datura.[4][5] Its Mandarin Chinese name 山莨菪碱 is given after Anisodus tanguticus (Chinese: 山莨菪; pinyin: shān làng dàng).[6]
- ^ Varma DR, Yue TL (March 1986). "Adrenoceptor blocking properties of atropine-like agents anisodamine and anisodine on brain and cardiovascular tissues of rats". British Journal of Pharmacology. 87 (3): 587–94. doi:10.1111/j.1476-5381.1986.tb10201.x. PMC 1916562. PMID 2879586.
- ^ "Pharmacopoeia Search: "山莨菪碱"". 中国药典. Archived from the original on 2017-12-03.
- ^ "消旋山莨菪碱滴眼液防治少年儿童假性近视的疗效分析". 国际医药卫生导报 (in Chinese (China)). 14 (15): 67–68. 2008. doi:10.3760/cma.j.issn.1007-1245.2008.15.027. Archived from the original on 2023-03-27. Retrieved 2017-12-03.
- ^ Ye N, Li J, Gao C, Xie Y (August 2013). "Simultaneous determination of atropine, scopolamine, and anisodamine in Flos daturae by capillary electrophoresis using a capillary coated by graphene oxide". Journal of Separation Science. 36 (16): 2698–702. doi:10.1002/jssc.201300304. PMID 23868645.
- ^ Zhang WW, Song MK, Cui YY, et al. (October 2008). "Differential neuropsychopharmacological influences of naturally occurring tropane alkaloids anisodamine versus scopolamine". Neuroscience Letters. 443 (3): 241–5. doi:10.1016/j.neulet.2008.07.048. PMID 18672024. S2CID 2730169.
- ^ "消旋山莨菪碱" (in Chinese (China)). 中国药典. Archived from the original on 2017-12-03. Retrieved 3 December 2017.