 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Apokyn, Kynmobi |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604020 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Subcutaneous injection (SQ), sublingual |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% following injection |
| Protein binding | ~50% |
| Metabolism | Liver, phase II |
| Onset of action | 10–20 min |
| Elimination half-life | 40 minutes |
| Duration of action | 60–90 min |
| Excretion | Liver |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.327 |
| Chemical and physical data | |
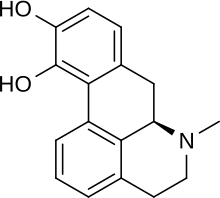
| Formula | C17H17NO2 |
| Molar mass | 267.328 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| Data page | |
| Apomorphine (data page) | |
| | |
Apomorphine, sold under the brand name Apokyn among others, is a type of aporphine having activity as a non-selective dopamine agonist which activates both D2-like and, to a much lesser extent, D1-like receptors.[2] It also acts as an antagonist of 5-HT2 and α-adrenergic receptors with high affinity. The compound is historically a morphine decomposition product made by boiling morphine with concentrated acid, hence the -morphine suffix. Contrary to its name, apomorphine does not actually contain morphine or its skeleton, nor does it bind to opioid receptors. The apo- prefix relates to it being a morphine derivative ("[comes] from morphine").
Historically, apomorphine has been tried for a variety of uses, including as a way to relieve anxiety and craving in alcoholics, an emetic (to induce vomiting), for treating stereotypies (repeated behaviour) in farmyard animals, and more recently in treating erectile dysfunction. Currently, apomorphine is used in the treatment of Parkinson's disease. It is a potent emetic and should not be administered without an antiemetic such as domperidone. The emetic properties of apomorphine are exploited in veterinary medicine to induce therapeutic emesis in canines that have recently ingested toxic or foreign substances.
Apomorphine was also used as a private treatment of heroin addiction, a purpose for which it was championed by the author William S. Burroughs. Burroughs and others claimed that it was a "metabolic regulator" with a restorative dimension to a damaged or dysfunctional dopaminergic system. Despite anecdotal evidence that this offers a plausible route to an abstinence-based mode, no clinical trials have ever tested this hypothesis. A recent study indicates that apomorphine might be a suitable marker for assessing central dopamine system alterations associated with chronic heroin consumption.[3] There is, however, no clinical evidence that apomorphine is an effective and safe treatment regimen for opiate addiction.[4]
- ^ "Movapo Pod (Stada Pharmaceuticals Australia Pty Ltd)". Therapeutic Goods Administration (TGA). 13 September 2024. Retrieved 15 September 2024.
- ^ Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes". The Journal of Pharmacology and Experimental Therapeutics. 303 (2): 791–804. doi:10.1124/jpet.102.039867. PMID 12388666. S2CID 6200455.
- ^ Guardia J, Casas M, Prat G, Trujols J, Segura L, Sánchez Turet M (October 2002). "The apomorphine test: a biological marker for heroin dependence disorder?". Addiction Biology. 7 (4): 421–426. doi:10.1080/1355621021000006206. PMID 14578019. S2CID 32386793.
- ^ Dent JY (1949). "Apomorphine Treatment of Addiction". British Journal of Addiction to Alcohol & Other Drugs. 46 (1): 15–28. doi:10.1111/j.1360-0443.1949.tb04502.x.