| |
| Names | |
|---|---|
| IUPAC name
Nitric acid trihydrochloride
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
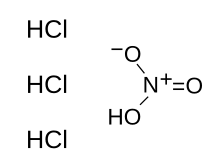
| HNO3 + 3 HCl | |
| Appearance | Fuming liquid. Freshly prepared aqua regia is colourless, but it turns yellow, orange or red within seconds. |
| Density | 1.01–1.21 g/cm3 |
| Melting point | −42 °C (−44 °F; 231 K) |
| Boiling point | 108 °C (226 °F; 381 K) |
| Miscible | |
| Vapor pressure | 21 mbar |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |


Aqua regia (/ˈreɪɡiə, ˈriːdʒiə/; from Latin, "regal water" or "royal water") is a mixture of nitric acid and hydrochloric acid, optimally in a molar ratio of 1:3.[b] Aqua regia is a fuming liquid. Freshly prepared aqua regia is colorless, but it turns yellow, orange or red within seconds from the formation of nitrosyl chloride and nitrogen dioxide. It was so named by alchemists because it can dissolve noble metals like gold and platinum, though not all metals.
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).
