| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Arsenate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| AsO3−4 | |||
| Molar mass | 138.918 g·mol−1 | ||
| Conjugate acid | Arsenic acid | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Extremely toxic, carcinogenic | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
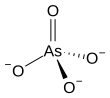
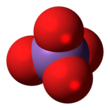
The arsenate is an ion with the chemical formula AsO3−4.[1] Bonding in arsenate consists of a central arsenic atom, with oxidation state +5, double bonded to one oxygen atom and single bonded to a further three oxygen atoms.[2] The four oxygen atoms orient around the arsenic atom in a tetrahedral geometry.[2] Resonance disperses the ion's −3 charge across all four oxygen atoms.
Arsenate readily reacts with metals to form arsenate metal compounds.[2][3] Arsenate is a moderate oxidizer and an electron acceptor, with an electrode potential of +0.56 V for its reduction to arsenite.[4] Due to arsenic having the same valency and similar atomic radius to phosphorus, arsenate shares similar geometry and reactivity with phosphate.[5] Arsenate can replace phosphate in biochemical reactions and is toxic to most organisms.[5][6]
- ^ PubChem. "Arsenate ion". pubchem.ncbi.nlm.nih.gov. Retrieved 2 April 2023.
- ^ a b c "Arsenate mineral | Britannica". www.britannica.com. Retrieved 2 April 2023.
- ^ Waalkes, Michael P. (2019), Baan, Robert A.; Stewart, Bernard W.; Straif, Kurt (eds.), "Arsenic and metals", Tumour Site Concordance and Mechanisms of Carcinogenesis, IARC Scientific Publications, Lyon (FR): International Agency for Research on Cancer, ISBN 978-92-832-2217-0, PMID 33979075, retrieved 2 April 2023
- ^ "P1: Standard Reduction Potentials by Element". Chemistry LibreTexts. 2 December 2013. Retrieved 29 March 2023.
- ^ a b Pollutants, National Research Council (US) Committee on Medical and Biological Effects of Environmental (1977). Chemistry of Arsenic. National Academies Press (US).
- ^ Elias, Mikael; Wellner, Alon; Goldin-Azulay, Korina; Chabriere, Eric; Vorholt, Julia A.; Erb, Tobias J.; Tawfik, Dan S. (2012). "The molecular basis of phosphate discrimination in arsenate-rich environments". Nature. 491 (7422): 134–137. Bibcode:2012Natur.491..134E. doi:10.1038/nature11517. ISSN 1476-4687. PMID 23034649. S2CID 99851438.