| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Arsenic(III) fluoride
| |||
| Other names
Arsenic trifluoride, trifluoroarsane, TL-156
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.029.145 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
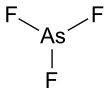
| AsF3 | |||
| Molar mass | 131.9168 g/mol | ||
| Appearance | colorless oily liquid | ||
| Density | 2.666 g/cm3 (0 °C)[1] | ||
| Melting point | −8.5 °C (16.7 °F; 264.6 K) | ||
| Boiling point | 60.4 °C (140.7 °F; 333.5 K) | ||
| decomposes | |||
| Solubility | soluble in alcohol, ether, benzene and ammonia solution | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Toxic, corrosive | ||
| GHS labelling: | |||

| |||
| Danger | |||
| H301, H311, H331 | |||
| P261, P264, P270, P271, P280, P301+P310, P302+P352, P304+P340, P311, P312, P321, P322, P330, P361, P363, P403+P233, P405, P501 | |||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
[1910.1018] TWA 0.010 mg/m3[2] | ||
REL (Recommended)
|
Ca C 0.002 mg/m3 [15-minute][2] | ||
IDLH (Immediate danger)
|
Ca [5 mg/m3 (as As)][2] | ||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
-821.3 kJ/mol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water.[3]
- ^ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0038". National Institute for Occupational Safety and Health (NIOSH).
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.