| |
| |
| Names | |
|---|---|
| IUPAC name
Arsorous acid
| |
| Other names
Arsenious acid
Arsenic oxide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H3AsO3 | |
| Molar mass | 125.94 g/mol |
| Appearance | Only exists in aqueous solutions |
| Conjugate base | Arsenite |
| -51.2·10−6 cm3/mol | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic, corrosive |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
[1910.1018] TWA 0.010 mg/m3[1] |
REL (Recommended)
|
Ca C 0.002 mg/m3 [15-minute][1] |
IDLH (Immediate danger)
|
Ca [5 mg/m3 (as As)][1] |
| Related compounds | |
Related compounds
|
Arsenic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
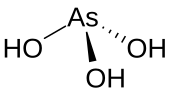
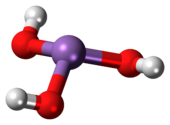
Arsenous acid (or arsenious acid) is the inorganic compound with the formula H3AsO3. It is known to occur in aqueous solutions, but it has not been isolated as a pure material, although this fact does not detract from the significance of As(OH)3.[2]