 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɑːrtɪˈmɪsɪnɪn/ |
| Other names | Artemisinine, qinghaosu |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.110.458 |
| Chemical and physical data | |
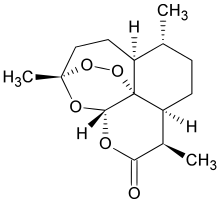
| Formula | C15H22O5 |
| Molar mass | 282.336 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.24 ± 0.1 g/cm3 |
| Melting point | 152 to 157 °C (306 to 315 °F) |
| Boiling point | decomposes |
| |
| |
| | |
Artemisinin (/ˌɑːrtɪˈmiːsɪnɪn/) and its semisynthetic derivatives are a group of drugs used in the treatment of malaria due to Plasmodium falciparum.[1] It was discovered in 1972 by Tu Youyou, who shared the 2015 Nobel Prize in Physiology or Medicine for her discovery.[2] Artemisinin-based combination therapies (ACTs) are now standard treatment worldwide for P. falciparum malaria as well as malaria due to other species of Plasmodium.[3] Artemisinin is extracted from the plant Artemisia annua (sweet wormwood) an herb employed in Chinese traditional medicine. A precursor compound can be produced using a genetically engineered yeast, which is much more efficient than using the plant.[4]
Artemisinin and its derivatives are all sesquiterpene lactones containing an unusual peroxide bridge. This endoperoxide 1,2,4-trioxane ring is responsible for their antimalarial properties. Few other natural compounds with such a peroxide bridge are known.[5]
Artemisinin and its derivatives have been used for the treatment of malarial and parasitic worm (helminth) infections. Advantages of such treatments over other anti-parasitics include faster parasite elimination and broader efficacy across the parasite life-cycle; disadvantages include their low bioavailability, poor pharmacokinetic properties, and high cost.[6][7] Moreover, use of the drug by itself as a monotherapy is explicitly discouraged by the World Health Organization,[8] as there have been signs that malarial parasites are developing resistance to the drug.[9] Combination therapies, featuring artemisinin or its derivatives alongside some other antimalarial drug, constitute the contemporary standard-of-care treatment regimen for malaria.[10]
- ^ Wang J, Xu C, Wong YK, Li Y, Liao F, Jiang T, et al. (2019). "Artemisinin, the Magic Drug Discovered from Traditional Chinese Medicine". Engineering. 5 (1): 32–9. Bibcode:2019Engin...5...32W. doi:10.1016/j.eng.2018.11.011.
- ^ Cite error: The named reference
nobel-2015was invoked but never defined (see the help page). - ^ WHO 2015, pp. 9–11.
- ^ Arsenault PR, Wobbe KK, Weathers PJ (2008). "Recent advances in artemisinin production through heterologous expression". Current Medicinal Chemistry. 15 (27): 2886–96. doi:10.2174/092986708786242813. PMC 2821817. PMID 18991643.
- ^ Brown G (2006). "Artemisinin and a new generation of antimalarial drugs". Education in Chemistry. 43 (4). Royal Society of Chemistry: 97–9. Retrieved 2018-03-09.
- ^ Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. (2012). "Pharmacogenomics knowledge for personalized medicine". Clinical Pharmacology and Therapeutics. 92 (4): 414–7. doi:10.1038/clpt.2012.96. PMC 3660037. PMID 22992668.
- ^ "Development of Novel Antimalarials". MalariaWorld. September 6, 2010. Retrieved 2016-10-22.
- ^ "WHO calls for an immediate halt to provision of single-drug artemisinin malaria pills". WHO. 19 January 2006. Archived from the original on November 16, 2006.
- ^ "Urgent action needed to stop spread of drug-resistant malaria". Voice of America. July 29, 2024.
- ^ Pelfrene E, Pinheiro MH, Cavaleri M (2015). "Artemisinin-based combination therapy in the treatment of uncomplicated malaria: review of recent regulatory experience at the European Medicines Agency". International Health. 7 (4): 239–46. doi:10.1093/inthealth/ihv017. PMC 4492341. PMID 25855638.