| Aspartate carbamoyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
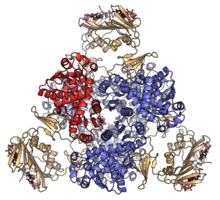 Escherichia coli aspartate carbamoyltransferase heterododecamer with catalytic subunits coloured red and blue, and regulatory subunits in orange. PDB: 4FYY | |||||||||
| Identifiers | |||||||||
| EC no. | 2.1.3.2 | ||||||||
| CAS no. | 9012-49-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Human carbamoyl-phosphate synthetase 2, aspartate transcarbamoylase, dihydroorotase | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | CAD | ||||||
| NCBI gene | 790 | ||||||
| HGNC | 1424 | ||||||
| OMIM | 114010 | ||||||
| RefSeq | NM_004341 | ||||||
| UniProt | P27708 | ||||||
| Other data | |||||||
| EC number | 2.1.3.2 | ||||||
| Locus | Chr. 2 p22-p21 | ||||||
| |||||||
Aspartate carbamoyltransferase (also known as aspartate transcarbamoylase or ATCase) catalyzes the first step in the pyrimidine biosynthetic pathway (EC 2.1.3.2).[1]
In E. coli, the enzyme is a multi-subunit protein complex composed of 12 subunits (300 kDa in total).[2] The composition of the subunits is C6R6, forming 2 trimers of catalytic subunits (34 kDa) and 3 dimers of regulatory subunits (17 kDa). The particular arrangement of catalytic and regulatory subunits in this enzyme affords the complex with strongly allosteric behaviour with respect to its substrates.[3] The enzyme is an archetypal example of allosteric modulation of fine control of metabolic enzyme reactions.
ATCase does not follow Michaelis–Menten kinetics. Instead, it lies between its low-activity, low-affinity "tense" and its high-activity, high-affinity "relaxed" states.[4] The binding of substrate to the catalytic subunits results in an equilibrium shift towards the R state, whereas binding of CTP to the regulatory subunits results in an equilibrium shift towards the T state. Binding of ATP to the regulatory subunits results in an equilibrium shift towards the R state.[5]
- ^ Simmer JP, Kelly RE, Rinker AG, Zimmermann BH, Scully JL, Kim H, Evans DR (Jan 1990). "Mammalian dihydroorotase: nucleotide sequence, peptide sequences, and evolution of the dihydroorotase domain of the multifunctional protein CAD". Proceedings of the National Academy of Sciences of the United States of America. 87 (1): 174–8. Bibcode:1990PNAS...87..174S. doi:10.1073/pnas.87.1.174. PMC 53223. PMID 1967494.
- ^ Macol CP, Tsuruta H, Stec B, Kantrowitz ER (May 2001). "Direct structural evidence for a concerted allosteric transition in Escherichia coli aspartate transcarbamoylase". Nature Structural Biology. 8 (5): 423–6. doi:10.1038/87582. PMID 11323717. S2CID 35403933.
- ^ Helmstaedt K, Krappmann S, Braus GH (Sep 2001). "Allosteric regulation of catalytic activity: Escherichia coli aspartate transcarbamoylase versus yeast chorismate mutase". Microbiology and Molecular Biology Reviews. 65 (3): 404–21, table of contents. doi:10.1128/MMBR.65.3.404-421.2001. PMC 99034. PMID 11528003.
- ^ Biochemistry, by Campbell and Farrel, Chapter 7
- ^ Alberts, Bruce. Molecular biology of the cell. ISBN 978-1-315-73536-8. OCLC 1082214404.