| Part of a series on the |
| Periodic table |
|---|
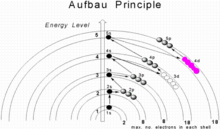 |
In atomic physics and quantum chemistry, the Aufbau principle (/ˈaʊfbaʊ/, from German: Aufbauprinzip, lit. 'building-up principle'), also called the Aufbau rule, states that in the ground state of an atom or ion, electrons first fill subshells of the lowest available energy, then fill subshells of higher energy. For example, the 1s subshell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration possible. An example is the configuration 1s2 2s2 2p6 3s2 3p3 for the phosphorus atom, meaning that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, and so on.
The configuration is often abbreviated by writing only the valence electrons explicitly, while the core electrons are replaced by the symbol for the last previous noble gas in the periodic table, placed in square brackets. For phosphorus, the last previous noble gas is neon, so the configuration is abbreviated to [Ne] 3s2 3p3, where [Ne] signifies the core electrons whose configuration in phosphorus is identical to that of neon.
Electron behavior is elaborated by other principles of atomic physics, such as Hund's rule and the Pauli exclusion principle. Hund's rule asserts that if multiple orbitals of the same energy are available, electrons will occupy different orbitals singly and with the same spin before any are occupied doubly. If double occupation does occur, the Pauli exclusion principle requires that electrons that occupy the same orbital must have different spins (+1⁄2 and −1⁄2).
Passing from one element to another of the next higher atomic number, one proton and one electron are added each time to the neutral atom. The maximum number of electrons in any shell is 2n2, where n is the principal quantum number. The maximum number of electrons in a subshell is equal to 2(2l + 1), where the azimuthal quantum number l is equal to 0, 1, 2, and 3 for s, p, d, and f subshells, so that the maximum numbers of electrons are 2, 6, 10, and 14 respectively. In the ground state, the electronic configuration can be built up by placing electrons in the lowest available subshell until the total number of electrons added is equal to the atomic number. Thus subshells are filled in the order of increasing energy, using two general rules to help predict electronic configurations:
- Electrons are assigned to subshells in order of increasing value of n + l.
- For subshells with the same value of n + l, electrons are assigned first to the subshell with lower n.
A version of the aufbau principle known as the nuclear shell model is used to predict the configuration of protons and neutrons in an atomic nucleus.[1]
- ^ Cottingham, W. N.; Greenwood, D. A. (1986). "Chapter 5: Ground state properties of nuclei: the shell model". An introduction to nuclear physics. Cambridge University Press. ISBN 0-521-31960-9.