| Steels |
|---|
 |
| Phases |
| Microstructures |
| Classes |
| Other iron-based materials |

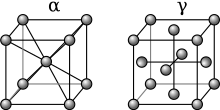
Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element.[1] In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902).[2] It exists at room temperature in some stainless steels due to the presence of nickel stabilizing the austenite at lower temperatures.
- ^ Reed-Hill R, Abbaschian R (1991). Physical Metallurgy Principles (3rd ed.). Boston: PWS-Kent Publishing. ISBN 978-0-534-92173-6.
- ^ Gove PB, ed. (1963). Webster's Seventh New Collegiate Dictionary. Springfield, Massachusetts, USA: G & C Merriam Company. p. 58.