 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌæzəˈθaɪəˌpriːn/[1] |
| Trade names | Azasan, Imuran, Jayempi, others |
| Other names | AZA |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682167 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60±31% |
| Protein binding | 20–30% |
| Metabolism | Activated non-enzymatically, deactivated mainly by xanthine oxidase |
| Elimination half-life | 26–80 minutes (azathioprine) 3–5 hours (drug plus metabolites) |
| Excretion | Kidney, 98% as metabolites |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.525 |
| Chemical and physical data | |
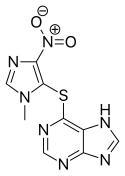
| Formula | C9H7N7O2S |
| Molar mass | 277.26 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 238 to 245 °C (460 to 473 °F) |
| |
| |
| (verify) | |
Azathioprine, sold under the brand name Imuran, among others, is an immunosuppressive medication.[5] It is used for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, Crohn's disease, ulcerative colitis, and systemic lupus erythematosus; and in kidney transplants to prevent rejection. It is listed by the International Agency for Research on Cancer as a group 1 human carcinogen.[5][6][7][8] It is taken by mouth or injected into a vein.[5]
Common side effects include bone-marrow suppression and vomiting.[5] Bone-marrow suppression is especially common in people with a genetic deficiency of the enzyme thiopurine S-methyltransferase.[5] Other serious risk factors include an increased risk of certain cancers.[5] Use during pregnancy may result in harm to the baby.[5] Azathioprine belongs to the purine analogues subclass of antimetabolites family of medications.[5][9] It works via 6-thioguanine to disrupt the making of RNA and DNA by cells.[5][9]
Azathioprine was first made in 1957.[9] It is on the World Health Organization's List of Essential Medicines.[10] In 2018, it was the 358th most commonly prescribed medication in the United States, with more than 800,000 prescriptions.[11]
- ^ "Azathioprine". Merriam-Webster.com Dictionary. Merriam-Webster.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Jayempi EPAR". European Medicines Agency. 20 April 2021. Retrieved 4 March 2023.
- ^ "Jayempi Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ a b c d e f g h i "Azathioprine". The American Society of Health-System Pharmacists. Archived from the original on 20 August 2016. Retrieved 8 December 2016.
- ^ Axelrad JE, Lichtiger S, Yajnik V (May 2016). "Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment". World Journal of Gastroenterology (Review). 22 (20): 4794–4801. doi:10.3748/wjg.v22.i20.4794. PMC 4873872. PMID 27239106.
- ^ Singer O, McCune WJ (May 2017). "Update on maintenance therapy for granulomatosis with polyangiitis and microscopic polyangiitis". Current Opinion in Rheumatology. 29 (3): 248–253. doi:10.1097/BOR.0000000000000382. PMID 28306595. S2CID 35805200.
- ^ Jordan N, D'Cruz D (2016). "Current and emerging treatment options in the management of lupus". ImmunoTargets and Therapy. 5: 9–20. doi:10.2147/ITT.S40675. PMC 4970629. PMID 27529058.
- ^ a b c Sami N (2016). Autoimmune Bullous Diseases: Approach and Management. Springer. p. 83. ISBN 9783319267289. Archived from the original on 2016-12-21.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Azathioprine - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.