| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
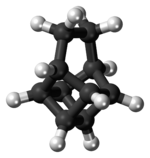
Pentacyclo[4.4.0.02,5.03,8.04,7]decane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H12 | |||
| Molar mass | 132.206 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Basketane is a polycyclic alkane with the chemical formula C10H12. The name is taken from its structural similarity to a basket shape. Basketane was first synthesized in 1966, independently[1] by Masamune[2] and Dauben and Whalen.[3] A patent application published in 1988 used basketane, which is a hydrocarbon, as a source material in doping thin diamond layers because of the molecule's high vapor pressure, carbon ring structure, and fewer hydrogen-to-carbon bond ratio.[4]
- ^ Marchand, A. P. (1989). "Synthesis and chemistry of homocubanes, bishomocubanes, and trishomocubanes". Chem. Rev. 89 (5): 1011–1033. doi:10.1021/cr00095a004.
- ^ Masamune, S.; Cuts, H.; Hogben, M. G. (1966). "Strained systems. VII. Pentacyclo[4.2.2.02,5.03,8.04,7]deca-9-ene, basketene". Tetrahedron Lett. 7 (10): 1017–1021. doi:10.1016/S0040-4039(00)70232-2.
- ^ Dauben, W. G.; Whalen, D. L. (1966). "Pentacyclo[4.4.0.02,5.03,8.04,7]decane and pentacyclo[4.3.0.02,5.03,8.04,7]nonane". Tetrahedron Lett. 7 (31): 3743–3750. doi:10.1016/S0040-4039(01)99958-7.
- ^ WO 1988002792, Pastor, Ricardo C., "Process for depositing layers of diamond", published 1988-04-21, assigned to Hughes Aircraft Co., since withdrawn.