 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cogentin, others |
| Other names | benzatropine (BAN UK), benztropine (USAN US) |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular, intravenous |
| Drug class | Antimuscarinic |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 12–24 hours |
| Duration of action | 10 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
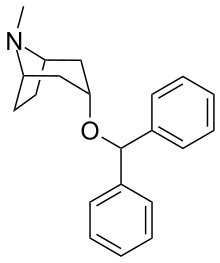
| Formula | C21H25NO |
| Molar mass | 307.437 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Benzatropine (INN),[2] known as benztropine in the United States and Japan,[3] is a medication used to treat movement disorders like parkinsonism and dystonia, as well as extrapyramidal side effects of antipsychotics, including akathisia.[4] It is not useful for tardive dyskinesia.[4] It is taken by mouth or by injection into a vein or muscle.[4] Benefits are seen within two hours and last for up to ten hours.[5][6]
Common side effects include dry mouth, blurry vision, nausea, and constipation.[4] Serious side effect may include urinary retention, hallucinations, hyperthermia, and poor coordination.[4] It is unclear if use during pregnancy or breastfeeding is safe.[7] Benzatropine is an anticholinergic which works by blocking the activity of muscarinic acetylcholine receptors.[4]
Benzatropine was approved for medical use in the United States in 1954.[4] It is available as a generic medication.[4] In 2020, it was the 229th most commonly prescribed medication in the United States, with more than 2 million prescriptions.[8][9] It is sold under the brand name Cogentin among others.[4]
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". Therapeutic Goods Administration (TGA). 21 June 2022. Archived from the original on 6 July 2023. Retrieved 30 March 2024.
- ^ World Health Organization (December 1959). "International Non-Proprietary Names for Pharmaceutical Preparations). Recommended International Non-Proprietary Names (Rec. I.N.N.): List 3º" (PDF). WHO Chronicle. 13 (12): 464. Archived (PDF) from the original on 28 August 2021. Retrieved 1 December 2020.
- ^ World Health Organization. "INN: Benzatropine". WHO MedNet. Retrieved 1 December 2020.[permanent dead link]
- ^ a b c d e f g h i "Benztropine Mesylate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 6 June 2019. Retrieved 9 April 2019.
- ^ Pagliaro LA, Pagliaro AM (1999). PNDR, Psychologists' Neuropsychotropic Drug Reference. Psychology Press. p. 47. ISBN 9780876309568.
- ^ Aschenbrenner DS, Venable SJ (2009). Drug Therapy in Nursing. Lippincott Williams & Wilkins. p. 197. ISBN 9780781765879.
- ^ "Benztropine (Cogentin) Use During Pregnancy". Drugs.com. Archived from the original on 6 June 2019. Retrieved 9 April 2019.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 12 February 2021. Retrieved 7 October 2022.
- ^ "Benztropine - Drug Usage Statistics". ClinCalc. Archived from the original on 12 May 2023. Retrieved 7 October 2022.