 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
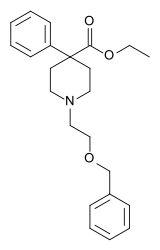
| Formula | C23H29NO3 |
| Molar mass | 367.489 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Benzethidine is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine, or Demerol).[2]
Benzethidine is not currently used in medicine and is a Class A/Schedule I drug which is controlled under UN drug conventions. It has similar effects to other opioid derivatives, such as analgesia, sedation, nausea and respiratory depression.[3] In the United States, the drug is a Schedule I Narcotic Controlled Substance with a DEA ACSCN of 9606 and 2014 annual aggregate manufacturing quota of nil.[4] The most common salt in use is the hydrochloride, free base conversion ratio of 0.910.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Maul C, Buschmann H, Sundermann B (2005). "Opioids: 3.3 Synthetic Opioids.". Analgesics. Wiley-VCH. pp. 159–169. ISBN 978-3-527-30403-5.
- ^ Cahal DA, Dare JG, Keith D (February 1961). "A sequential trial of analgesics in labour". The Journal of Obstetrics and Gynaecology of the British Commonwealth. 68: 88–93. doi:10.1111/j.1471-0528.1961.tb02689.x. PMID 13689779. S2CID 27397119.
- ^ "Conversion Factors for Controlled Substances". Diversion Control Division. Drug Enforcement Agency, U.S. Department of Justice.