 | |
 | |
| Clinical data | |
|---|---|
| Other names | KP201 |
| Routes of administration | Oral |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
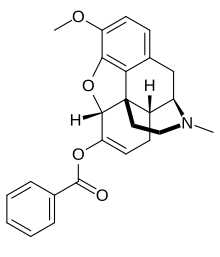
| Formula | C25H25NO4 |
| Molar mass | 403.478 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Benzhydrocodone (INN) (contracted from benzoate-hydrocodone) is an opioid prodrug of the morphinan class. Its chemical structure consists of hydrocodone coupled with benzoic acid. Benzhydrocodone itself is inactive and acts as a prodrug to hydrocodone upon cleavage of the benzoate portion of the molecule.[1]
It is designed to be an opioid analgesic with a low chance of recreational use.[2]
Created by Kempharm, Inc., a biopharmaceutical company in Coralville, Iowa, President and CEO, Travis Mickle, believes the molecular-based approach to abuse deterrent may be more effective than many formulation-based approaches.[3][4]
When approved, Apadaz received a labeling that highlighted all relevant aspects of the drug, including the lower abuse profile compared to traditional hydrocodone-acetaminophen. The labeling showed several items supporting a lower abuse profile than traditional hydrocodone-acetaminophen; namely, a lower Drug Liking in the first two hours after intranasal abuse (snorting), and the conversion of benzhydrocodone to hydrocodone in vitro being a "difficult process" — with benzhydrocodone being a more difficult drug to abuse according to FDA advisory committee documents.[5][6]
- ^ KemPharm, Inc. (June 11, 2013). "KemPharm, Inc. Receives Patent from the USPTO for Novel Pain Drug Candidate, KP201" (Press release). North Liberty, Iowa: PR Newswire. Retrieved February 8, 2015.
- ^ Lavitt J (June 12, 2014). "New Abuse-Resistant Opioid Receives $60 Million Backing". thefix.com/. The Fix. Retrieved February 8, 2015.
- ^ "KP201/APAP". kempharm.com/. Retrieved April 20, 2015.
- ^ Cite error: The named reference
:0was invoked but never defined (see the help page). - ^ "APADAZ (benzhydrocodone and acetaminophen) tablets, for oral use, CII" (PDF). KemPharm, Inc. U.S. Food and Drug Administration.
- ^ "2016 Meeting Materials, Anesthetic and Analgesic Drug Products Advisory Committee". U.S. Food and Drug Administration.