| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Phenylmethanol (Hydroxymethyl)benzene | |
| Other names
Benzyl alcohol
α-Cresol α-Toluenol α-Hydroxytoluene alpha-Hydroxyphenylmethane Phenylcarbinol Benzenemethanol Benzyl hydroxide Benzylic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.600 |
| EC Number |
|
| E number | E1519 (additional chemicals) |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[1] | |
| C7H8O | |
| Molar mass | 108.140 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Slightly aromatic |
| Density | 1.044 g/cm3 |
| Melting point | −15.2 °C (4.6 °F; 257.9 K) |
| Boiling point | 205.3 °C (401.5 °F; 478.4 K) |
| 3.50 g/100 mL (20 °C) 4.29 g/100 mL (25 °C) | |
| Solubility in other solvents | Miscible with benzene, methanol, chloroform, ethanol, ether, acetone |
| log P | 1.10 |
| Vapor pressure | 0.18 kPa (60 °C) |
| Acidity (pKa) | 15.40 |
| −71.83·10−6 cm3/mol | |
Refractive index (nD)
|
1.5396 |
| Viscosity | 5.474 cP |
| 1.67 D | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
217.8 J/(K·mol) |
Std enthalpy of
formation (ΔfH⦵298) |
−352 kJ/mol |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 93 °C (199 °F; 366 K) |
| 436 °C (817 °F; 709 K) | |
| Explosive limits | 1.3–13% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1250 mg/kg (rat, oral) |
| Safety data sheet (SDS) | External MSDS |
| Pharmacology | |
| P03AX06 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
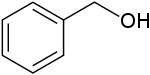
Benzyl alcohol (also known as α-cresol) is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is useful as a solvent for its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide.
- ^ "Benzyl alcohol". Archived from the original on 26 July 2009.
