| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.032.740 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UN number | 1566 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
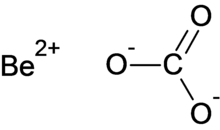
| BeCO3 | |
| Molar mass | 69.020 g·mol−1 |
| Melting point | 54 °C (129 °F; 327 K) |
| Boiling point | 100 °C (212 °F; 373 K) decomposes |
| 0.36 g/100 mL | |
| Thermochemistry | |
Heat capacity (C)
|
65 J/mol·K[1] |
Std molar
entropy (S⦵298) |
52 J/mol·K[1] |
Std enthalpy of
formation (ΔfH⦵298) |
-1025 kJ/mol[1] |
Gibbs free energy (ΔfG⦵)
|
-948 kJ/mol[1] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic (T) Irritant (Xi) |
| GHS labelling:[3] | |
  
| |
| Danger | |
| H301, H315, H317, H319, H330, H335, H350i, H372, H411 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
150 mg/kg (guinea pig) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[2] |
REL (Recommended)
|
Ca C 0.0005 mg/m3 (as Be)[2] |
IDLH (Immediate danger)
|
Ca [4 mg/m3 (as Be)][2] |
| Related compounds | |
Other cations
|
Magnesium carbonate Calcium carbonate Strontium carbonate Barium carbonate Radium carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Beryllium carbonate is a chemical compound with the chemical formula BeCO3.
- ^ a b c d "Beryllium carbonate".
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0054". National Institute for Occupational Safety and Health (NIOSH).
- ^ GHS: GESTIS 082790
