 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Celestone, Eleuphrat, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682799 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, topical, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver CYP3A4 |
| Elimination half-life | 36-54 hours |
| Excretion | Kidney (in urine) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.206 |
| Chemical and physical data | |
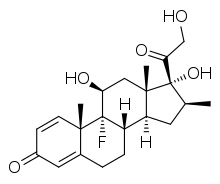
| Formula | C22H29FO5 |
| Molar mass | 392.467 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Betamethasone is a steroid medication.[3] It is used for a number of diseases including rheumatic disorders such as rheumatoid arthritis and systemic lupus erythematosus, skin diseases such as dermatitis and psoriasis, allergic conditions such as asthma and angioedema, preterm labor to speed the development of the baby's lungs, Crohn's disease, cancers such as leukemia, and along with fludrocortisone for adrenocortical insufficiency, among others.[3] It can be taken by mouth, injected into a muscle, or applied to the skin, typically in cream, lotion, or liquid forms.[3][4]
Serious side effects include an increased risk of infection, muscle weakness, severe allergic reactions, and psychosis.[3] Long-term use may cause adrenal insufficiency.[3] Stopping the medication suddenly following long-term use may be dangerous.[3] The cream commonly results in increased hair growth and skin irritation.[4] Betamethasone belongs to the glucocorticoid class of medication.[3] It is a stereoisomer of dexamethasone, the two compounds differing only in the spatial configuration of the methyl group at position 16 (see steroid nomenclature).[5]
Betamethasone was patented in 1958, and approved for medical use in the United States in 1961.[3][6] The cream and ointment are on the World Health Organization's List of Essential Medicines.[7] It is available as a generic medication.[3] In 2021, it was the 251st most commonly prescribed medication in the United States, with more than 1 million prescriptions.[8][9]
- ^ "Betamethasone Use During Pregnancy". Drugs.com. 30 December 2019. Retrieved 29 March 2020.
- ^ "Betamethasone 500 microgram Soluble Tablets - Summary of Product Characteristics (SmPC)". (emc). 5 April 2018. Retrieved 29 March 2020.
- ^ a b c d e f g h i "Betamethasone". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 2 December 2015.
- ^ a b "Betamethasone topical". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 2 December 2015.
- ^ Antignac JP, Le Bizec B, Monteau F, Andre F (January 2002). "Differentiation of betamethasone and dexamethasone using liquid chromatography/positive electrospray tandem mass spectrometry and multivariate statistical analysis". Journal of Mass Spectrometry. 37 (1): 69–75. Bibcode:2002JMSp...37...69A. doi:10.1002/jms.260. PMID 11813313.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 485. ISBN 9783527607495.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Betamethasone - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.