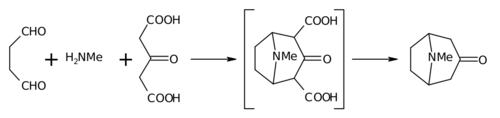
Biomimetic synthesis is an area of organic chemical synthesis that is specifically biologically inspired. The term encompasses both the testing of a "biogenetic hypothesis" (conjectured course of a biosynthesis in nature) through execution of a series of reactions designed to parallel the proposed biosynthesis, as well as programs of study where a synthetic reaction or reactions aimed at a desired synthetic goal are designed to mimic one or more known enzymic transformations of an established biosynthetic pathway.[1][2] The earliest generally cited example of a biomimetic synthesis is Sir Robert Robinson's organic synthesis of the alkaloid tropinone.[3]
A more recent example is E.J. Corey's carbenium-mediated cyclization of an engineered linear polyene to provide a tetracyclic steroid ring system,[4] which built upon studies of cationic cyclizations of linear polyenes by the Albert Eschenmoser and Gilbert Stork,[5][6] and the extensive studies of the W.S. Johnson to define the requirements to initiate and terminate the cyclization, and to stabilize the cationic carbenium group during the cyclization (as nature accomplishes via enzymes during biosynthesis of steroids such as cholesterol).[7] In relation to the second definition, synthetic organic or inorganic catalysts applied to accomplish a chemical transformation accomplished in nature by a biocatalyst (e.g., a purely proteinaceous catalyst, a metal or other cofactor bound to an enzyme, or a ribozyme) can be said to be accomplishing a biomimetic synthesis, where design and characterization of such catalytic systems has been termed biomimetic chemistry.[8][9][10]
- ^ de la Torre MC, Sierra MA (January 2004). "Comments on recent achievements in biomimetic organic synthesis". Angew. Chem. Int. Ed. Engl. 43 (2): 160–81. doi:10.1002/anie.200200545. PMID 14695603.
- ^ van Tamelen EE (1961). "Biogenetic-type Syntheses of Natural Products". Fortschritte der Chemie Organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products / Progrès dans la Chimie des Substances Organiques Naturelles. Vol. 19. pp. 242–290. doi:10.1007/978-3-7091-7156-1_5. ISBN 978-3-7091-7158-5. PMID 13924635.
- ^ Robinson R (1917). "LXIII. A Synthesis of Tropinone". Journal of the Chemical Society, Transactions. 111: 762–768. doi:10.1039/CT9171100762.
- ^ Corey EJ, Luo G, Lin LS (1997). "A simple enantioselective synthesis of the biologically active tetracyclic marine sesterterpene scalarenedial". J. Am. Chem. Soc. 119 (41): 9927–28. doi:10.1021/ja972690l.
- ^ Eschenmoser A, Felix D, Gut M, Meier J, Stadler P (1959). "Some aspects of acid-catalysed cyclizations of terpenoid polyenes". In Wolstenholme GE, O'Conner M (eds.). Ciba Foundation Symposium on the Biosynthesiis of Terpenes and Steroids. Novartis Foundation Symposia. London: J. & A. Churchill. pp. 217–230. doi:10.1002/9780470719121.ch14. ISBN 9780470719121.
- ^ Stork G, Burgstrahler AW (1955). "The stereochemistry of polyene cyclization". J. Am. Chem. Soc. 77 (19): 5068–77. doi:10.1021/ja01624a038.
- ^ Johnson WS, Marshall JA, Keana JF, Franck RW, Martin DG, Bauer JV (1966). "Steroid total synthesis—hydrochrysene approach—XVI: Racemic conessine, progesterone, cholesterol, and some related natural products". Tetrahedron. 22: 541–601. doi:10.1016/S0040-4020(01)90961-5.
- ^ Breslow R (January 2009). "Biomimetic chemistry: biology as an inspiration". The Journal of Biological Chemistry. 284 (3): 1337–42. doi:10.1074/jbc.X800011200. PMID 18784073.
- ^ Lee SC, Holm RH (April 2003). "Speculative synthetic chemistry and the nitrogenase problem". Proceedings of the National Academy of Sciences of the United States of America. 100 (7): 3595–600. doi:10.1073/pnas.0630028100. PMC 152967. PMID 12642670.
- ^ Breslow R (1995). "Biomimetic chemistry and artificial enzymes: Catalysis by design". Accounts of Chemical Research. 28 (3): 146–153. doi:10.1021/ar00051a008.