 | |
 | |
| Clinical data | |
|---|---|
| Other names | Brallobarbital |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.008.387 |
| Chemical and physical data | |
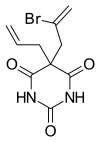
| Formula | C10H11BrN2O3 |
| Molar mass | 287.113 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Brallobarbital was a barbiturate developed in the 1920s.[1] It has sedative and hypnotic properties, and was used for the treatment of insomnia. Brallobarbital was primarily sold as part of a combination product called Vesparax, composed of 150 mg secobarbital, 50 mg brallobarbital and 50 mg hydroxyzine.[2] The long half-life of this combination of drugs tended to cause a hangover effect the next day,[3] and Vesparax fell into disuse once newer drugs with lesser side effects had been developed. Vesparax reportedly was the drug that musician Jimi Hendrix supposedly overdosed on and led to his untimely death. It is no longer made.[4]
- ^ US 1869666
- ^ Lhermann J (March 1964). "Clinical application of a new very active hypnotic associating sodium secobarbital, calcium brallobarbital and hydroxyzine (UC-8130)". Gazette Médicale de France (in French). 71: 961–2. PMID 14142825.
- ^ Yih TD, Rossum JM (June 1976). "Peculiar pharmacokinetics of brallobarbital as a source of complications in Vesparax intoxication". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 6 (6): 355–62. doi:10.3109/00498257609151647. PMID 969563.
- ^ Fischbach R (February 1983). "Efficacy and safety of midazolam and vesparax in treatment of sleep disorders". British Journal of Clinical Pharmacology. 16 (S1): 167S–171S. doi:10.1111/j.1365-2125.1983.tb02290.x. PMC 1428085. PMID 6138072.