This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
Brevetoxin (PbTx), or brevetoxins, are a suite of cyclic polyether compounds produced naturally by a species of dinoflagellate known as Karenia brevis. Brevetoxins are neurotoxins that bind to voltage-gated sodium channels in nerve cells, leading to disruption of normal neurological processes and causing the illness clinically described as neurotoxic shellfish poisoning (NSP).[1]
Although brevetoxins are most well-studied in K. brevis, they are also found in other species of Karenia and at least one large fish kill has been traced to brevetoxins in Chattonella.[1]
| Brevetoxin A[2] | Brevetoxin B[3] | |
|---|---|---|
| chemical structure | 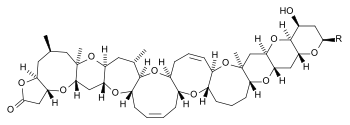 |
 |
| subtypes |
|
|
Other Brevetoxins:
- Brevetoxin-5 (PbTx-5): like PbTx-2, but acetylated hydroxyl group in position 38.
- Brevetoxin-6 (PbTx-6): like PbTx-2, but double bond 27-28 is epoxidated.
Brevetoxin-B was synthesized in 1995 by K. C. Nicolaou and coworkers in 123 steps with 91% average yield (final yield ~9·10−6)[4] and in 2004 in a total of 90 steps with an average 93% yield for each step (0.14% overall).[3]
K. C. Nicolaou and coworkers reported their synthesis of Brevetoxin-1 in 1998.[5] In 2009, Michael Crimmins and co-workers reported their synthesis of Brevetoxin-1 as well.[6]
- ^ a b Watkins SM, Reich A, Fleming LE, Hammond R (2008). "Neurotoxic Shellfish Poisoning". Marine Drugs. 6 (3): 431–455. doi:10.3390/md20080021. PMC 2579735. PMID 19005578.
- ^ Nicolaou KC, Yang Z, Shi G, Gunzner JL, Agrios KA, Gärtner P (1998). "Total Synthesis of Brevetoxin A". Nature. 392 (6673): 264–269. Bibcode:1998Natur.392..264N. doi:10.1038/32623. PMID 9521320. S2CID 373710.
- ^ a b Matsuo G, Kawamura K, Hori N, Matsukura H, Nakata T (2004). "Total Synthesis of Brevetoxin-B". Journal of the American Chemical Society. 126 (44): 14374–14376. doi:10.1021/ja0449269. PMID 15521755.
- ^ Nicolaou KC, Rutjes FP, Theodorakis EA, Tiebes J, Sato M, Untersteller E (1995). "Total Synthesis of Brevetoxin B. 3. Final Strategy and Completion". Journal of the American Chemical Society. 117 (41): 10252–10263. doi:10.1021/ja00146a010. hdl:2066/26297.
- ^ Nicolaou KC, Yang Z, Shi GQ, Gunzner JL, Agrios KA, Gärtner P (1998). "Total Synthesis of Brevetoxin A". Nature. 392 (6673): 264–269. Bibcode:1998Natur.392..264N. doi:10.1038/32623. PMID 9521320. S2CID 373710.
- ^ Crimmins MT, Zuccarello JL, Ellis JM, McDougall PJ, Haile PA, Parrish JD, Emmitte KA (2009). "Total Synthesis of Brevetoxin A". Organic Letters. 11 (2): 489–492. doi:10.1021/ol802710u. PMC 2640830. PMID 19099481.