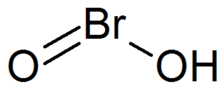
| |

| |

| |
| Names | |
|---|---|
| IUPAC names
hydroxy-λ3-bromanone
hydroxidooxidobromine bromous acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| HBrO2 | |
| Molar mass | 112.911 g/mol |
| Conjugate base | Bromite |
| Related compounds | |
Other anions
|
Hydrobromic acid; hypobromous acid; bromic acid; perbromic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bromous acid is the inorganic compound with the formula of HBrO2. It is an unstable compound, although salts of its conjugate base – bromites – have been isolated. In acidic solution, bromites decompose to bromine.[1]
- ^ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5