 | |
| Clinical data | |
|---|---|
| Trade names | Bromfed, Dimetapp, Bromfenex, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682545 |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 24.9 ± 9.3 hours[1] |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.507 |
| Chemical and physical data | |
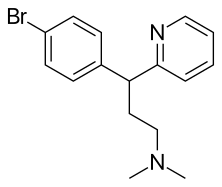
| Formula | C16H19BrN2 |
| Molar mass | 319.246 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Brompheniramine, sold under the brand name Dimetapp among others, is a first-generation antihistamine drug of the propylamine (alkylamine) class.[2] It is indicated for the treatment of the symptoms of the common cold and allergic rhinitis, such as runny nose, itchy eyes, watery eyes, and sneezing. Like the other first-generation drugs of its class, it is considered a sedating antihistamine.[2]
It was patented in 1948 and came into medical use in 1955.[3] In 2021, the combination with dextromethorphan and pseudoephedrine was the 294th most commonly prescribed medication in the United States with more than 500,000 prescriptions.[4][5]
- ^ Simons FE, Frith EM, Simons KJ (December 1982). "The pharmacokinetics and antihistaminic effects of brompheniramine". The Journal of Allergy and Clinical Immunology. 70 (6): 458–64. doi:10.1016/0091-6749(82)90009-4. PMID 6128358.
- ^ a b Sweetman SC, ed. (2005). Martindale: the complete drug reference (34th ed.). London: Pharmaceutical Press. p. 569–70. ISBN 0-85369-550-4. OCLC 56903116.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 546. ISBN 9783527607495.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Brompheniramine; Dextromethorphan; Pseudoephedrine - Drug Usage Statistics". ClinCalc. Retrieved 14 January 2024.