 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lendormin |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 48–95% |
| Metabolism | Hepatic |
| Elimination half-life | 4.4 hours (range, 2.6–6.9 h) |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.055.404 |
| Chemical and physical data | |
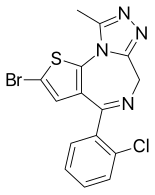
| Formula | C15H10BrClN4S |
| Molar mass | 393.69 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Brotizolam[3] (marketed under brand name Lendormin) is a sedative-hypnotic[4] thienotriazolodiazepine[5] drug which is a benzodiazepine analog.[6] It possesses anxiolytic, anticonvulsant, hypnotic, sedative and skeletal muscle relaxant properties, and is considered to be similar in effect to other short-acting hypnotic benzodiazepines such as triazolam or midazolam.[7] It is used in the short-term treatment of severe insomnia. Brotizolam is a highly potent and short-acting hypnotic, with a typical dose ranging from 0.125 to 0.25 milligrams, which is rapidly eliminated with an average half-life of 4.4 hours (range 3.6–7.9 hours).[8]
It was patented in 1974[9] and came into medical use in 1984.[10] Brotizolam is not approved for sale in the UK, United States or Canada. It is approved for sale in the Netherlands, Germany, Spain, Belgium, Luxembourg, Austria, Portugal, Israel, Italy, Taiwan and Japan.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ "DEA Diversion Control Division" (PDF). Archived from the original (PDF) on 2019-10-17. Retrieved 2015-12-10.
- ^ US 4094984 6-Phenyl-8-bromo-4H-s-triazolo-[3,4C]-thieno-[2,3E]-1,4-diazepines and salts thereof
- ^ Fink M, Irwin P (September 1981). "Pharmacoelectroencephalographic study of brotizolam, a novel hypnotic". Clinical Pharmacology and Therapeutics. 30 (3): 336–42. doi:10.1038/clpt.1981.169. PMID 7273596. S2CID 30788171.
- ^ Catabay A, Taniguchi M, Jinno K, Pesek JJ, Williamsen E (1 March 1998). "Separation of 1,4-Benzodiazepines and Analogues Using Cholesteryl-10-Undecenoate Bonded Phase in Microcolumn Liquid Chromatography". Journal of Chromatographic Science. 36 (3): 113. doi:10.1093/chromsci/36.3.111.
- ^ Jochemsen R, Wesselman JG, van Boxtel CJ, Hermans J, Breimer DD (1983). "Comparative pharmacokinetics of brotizolam and triazolam in healthy subjects". British Journal of Clinical Pharmacology. 16 (Suppl 2): 291S–297S. doi:10.1111/j.1365-2125.1983.tb02303.x. PMC 1428224. PMID 6140948.
- ^ Mandrioli R, Mercolini L, Raggi MA (October 2008). "Benzodiazepine metabolism: an analytical perspective". Current Drug Metabolism. 9 (8): 827–44. doi:10.2174/138920008786049258. PMID 18855614.
- ^ Langley MS, Clissold SP (February 1988). "Brotizolam. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy as an hypnotic". Drugs. 35 (2): 104–22. doi:10.2165/00003495-198835020-00002. PMID 3281819. S2CID 243228878.
- ^ US4094984 6-Phenyl-8-bromo-4H-s-triazolo-[3,4C]-thieno-[2,3E]-1,4-diazepines and salts thereof
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 539. ISBN 9783527607495.