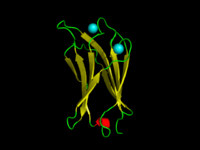
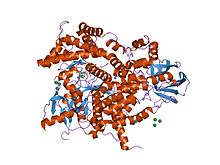
Phosphoinositide 3-kinase C2 Structure of phosphoinositide 3-kinase.
[ 1] Symbol PI3K_C2 Pfam PF00792 Pfam clanCL0154 ECOD 11.2.1 InterPro IPR002420 SMART PI3K_C2 PROSITE PDOC50004 SCOP2 1e8x / SCOPe / SUPFAM CDD cd08380 Pfam
structures / ECOD
PDB RCSB PDB ; PDBe ; PDBj PDBsum structure summary PDB 1e7u 1e7v 1e8w 1e8x 1e8y 1e8z 1e90 1he8 2a4z 2a5u 2chw 2chx 2chz 2rd0 2v4l 3csf 3cst 3dbs 3dpd 3ene
A C2 domain is a protein structural domain involved in targeting proteins to cell membranes . The typical version (PKC-C2) has a beta-sandwich composed of 8 β-strands that co-ordinates two or three calcium ions, which bind in a cavity formed by the first and final loops of the domain, on the membrane binding face. Many other C2 domain families don't have calcium binding activity.[ 2] [ 3]
^ Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL (October 2000). "Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine" . Molecular Cell . 6 (4): 909–19. doi :10.1016/S1097-2765(05)00089-4 PMID 11090628 . ^ Zhang D, Aravind L (December 2010). "Identification of novel families and classification of the C2 domain superfamily elucidate the origin and evolution of membrane targeting activities in eukaryotes" . Gene . 469 (1–2): 18–30. doi :10.1016/j.gene.2010.08.006 . PMC 2965036 PMID 20713135 . ^ Zhang D, Aravind L (October 2012). "Novel transglutaminase-like peptidase and C2 domains elucidate the structure, biogenesis and evolution of the ciliary compartment" . Cell Cycle . 11 (20): 3861–75. doi :10.4161/cc.22068 . PMC 3495828 PMID 22983010 .