 | |
| Clinical data | |
|---|---|
| Trade names | Jevtana |
| Other names | XRP-6258 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.205.741 |
| Chemical and physical data | |
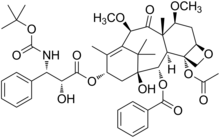
| Formula | C45H57NO14 |
| Molar mass | 835.944 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cabazitaxel, sold under the brand name Jevtana, is a semi-synthetic derivative of a natural taxoid.[4] It is a microtubule inhibitor,[2] and the fourth taxane to be approved as a cancer therapy.[citation needed]
Cabazitaxel was developed by Sanofi-Aventis and was approved by the US Food and Drug Administration (FDA) for the treatment of hormone-refractory prostate cancer in June 2010.[5][6][7] It is available as a generic medication.[8][9]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved October 22, 2023.
- ^ a b "Jevtana- cabazitaxel kit". DailyMed. Retrieved December 30, 2021.
- ^ "Jevtana EPAR". European Medicines Agency (EMA). March 17, 2011. Retrieved August 10, 2024.
- ^ "Cabazitaxel". NCI Drug Dictionary. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. February 2, 2011.
- ^ "Drug Approval Package: Jevtana (Cabazitaxel) NDA #201023". U.S. Food and Drug Administration (FDA). July 8, 2013. Retrieved December 30, 2021.
- ^ "Jevtana (cabazitaxel) Injection Approved by U.S. FDA After Priority Review" (Press release). Sanofi Aventis. June 17, 2010. Retrieved December 30, 2021 – via PR Newswire.
- ^ "Jevtana (cabazitaxel) Injection Approved by U.S. FDA After Priority Review - Jun 17, 2010" (Press release). Sanofi. June 17, 2010. Retrieved December 30, 2021.
- ^ "Cabazitaxel: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved December 30, 2021.
- ^ "First Generic Drug Approvals". U.S. Food and Drug Administration. October 17, 2022. Retrieved November 28, 2022.