| |

| |
| Names | |
|---|---|
| IUPAC name
Selanylidenecadmium[2]
| |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.772 |
| EC Number |
|
| 13656 | |
| MeSH | cadmium+selenide |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2570 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CdSe | |
| Molar mass | 191.385 g·mol−1 |
| Appearance | Black, translucent, adamantine crystals |
| Odor | Odorless |
| Density | 5.81 g cm−3[3] |
| Melting point | 1,240 °C (2,260 °F; 1,510 K)[3] |
| Band gap | 1.74 eV, both for hex. and sphalerite[4] |
Refractive index (nD)
|
2.5 |
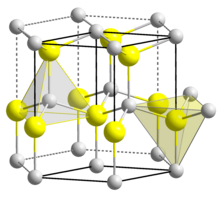
| Structure | |
| Wurtzite | |
| C6v4-P63mc | |
| Hexagonal | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H301, H312, H331, H373, H410 | |
| P261, P273, P280, P301+P310, P311, P501 | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
[1910.1027] TWA 0.005 mg/m3 (as Cd)[5] |
REL (Recommended)
|
Ca[5] |
IDLH (Immediate danger)
|
Ca [9 mg/m3 (as Cd)][5] |
| Related compounds | |
Other anions
|
Cadmium oxide, Cadmium sulfide, Cadmium telluride |
Other cations
|
Zinc selenide, Mercury(II) selenide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cadmium selenide is an inorganic compound with the formula CdSe. It is a black to red-black solid that is classified as a II-VI semiconductor of the n-type. It is a pigment, but applications are declining because of environmental concerns.[6]
- ^ a b "cadmium selenide (CHEBI:50834)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. IUPAC Names.
- ^ "cadmium selenide – PubChem Public Chemical Database". The PubChem Project. USA: Nation Center for Biotechnology Information. Descriptors Computed from Structure.
- ^ a b Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.54. ISBN 1-4398-5511-0.
- ^ Ninomiya, Susumu; Adachi, Sadao (1995). "Optical properties of cubic and hexagonal Cd Se". Journal of Applied Physics. 78 (7): 4681. Bibcode:1995JAP....78.4681N. doi:10.1063/1.359815.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0087". National Institute for Occupational Safety and Health (NIOSH).
- ^ Langner, Bernd E. (2000). "Selenium and Selenium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a23_525. ISBN 3527306730.