 | |
 | |
| Combination of | |
|---|---|
| Calcipotriol | Vitamin D3 analog |
| Betamethasone dipropionate | Corticosteroid |
| Clinical data | |
| Trade names | Taclonex, Enstilar, Dovobet, others |
| AHFS/Drugs.com | Professional Drug Facts |
| Pregnancy category |
|
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| KEGG | |
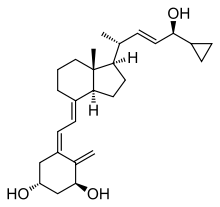
Calcipotriol/betamethasone dipropionate, sold under the brand name Taclonex among others, is a fixed-dose combination medication of the synthetic vitamin D3 analog calcipotriol (also known as calcipotriene) and the synthetic corticosteroid betamethasone dipropionate for the treatment of plaque psoriasis.[6][7][8] It is used in the form of ointment, topical suspension, gel, aerosol, and foam.[5][6][7][8]
It is available as a generic medication.[9][10]
- ^ "TGA eBS - Product and Consumer Medicine Information Licence".
- ^ "Glenbex Calcipotriol (as monohydrate) 50 microgram/g and Betamethasone (as dipropionate) 500 microgram/g Foam Aerosol Can (386430)". Therapeutic Goods Administration (TGA). 3 June 2023. Retrieved 10 September 2023.
- ^ "Approved in 2020: Drugs for human use". Health Canada. 26 July 2021. Retrieved 27 March 2024.
- ^ "Dovobet Ointment - Summary of Product Characteristics (SmPC)". (emc). 14 August 2018. Retrieved 19 October 2020.
- ^ a b "Dovobet gel - Summary of Product Characteristics (SmPC)". (emc). 1 October 2019. Retrieved 19 October 2020.
- ^ a b c "Taclonex- calcipotriene and betamethasone dipropionate ointment". DailyMed. 21 May 2020. Retrieved 19 October 2020.
- ^ a b c "Taclonex- calcipotriene and betamethasone dipropionate suspension". DailyMed. 16 June 2020. Retrieved 19 October 2020.
- ^ a b c "Enstilar- calcipotriene and betamethasone dipropionate aerosol, foam". DailyMed. 10 August 2020. Retrieved 19 October 2020.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- ^ "First Generic Drug Approvals 2023". U.S. Food and Drug Administration (FDA). 30 May 2023. Archived from the original on 30 June 2023. Retrieved 30 June 2023.