| |
 Calcium acetate crystals
| |
| Names | |
|---|---|
| Preferred IUPAC name
Calcium diacetate[1] | |
| Other names
Acetate of lime
Calcium ethanoate | |
| Identifiers | |
3D model (JSmol)
|
|
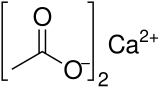
| Abbreviations | Ca(OAc)2 |
| 3692527 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.492 |
| EC Number |
|
| E number | E263 (preservatives) |
| 22320 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H6CaO4 | |
| Molar mass | 158.166 g/mol (anhydrous) 176.181 g/mol (monohydrate) |
| Appearance | White solid hygroscopic |
| Odor | slight acetic acid odor |
| Density | 1.509 g/cm3 |
| Melting point | 160 °C (320 °F; 433 K)[2] decomposition to CaCO3 + acetone |
| 37.4 g/100 mL (0 °C) 34.7 g/100 mL (20 °C) 29.7 g/100 mL (100 °C) | |
| Solubility | slightly soluble in methanol, hydrazine insoluble in acetone, ethanol and benzene |
| Acidity (pKa) | ca. 0.7 |
| -70.7·10−6 cm3/mol | |
Refractive index (nD)
|
1.55 |
| Pharmacology | |
| V03AE07 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| 680 to 730 °C (1,256 to 1,346 °F; 953 to 1,003 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
4280 mg/kg (oral, rat) |
| Related compounds | |
Other cations
|
Magnesium acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous form is very hygroscopic; therefore the monohydrate (Ca(CH3COO)2•H2O) is the common form.
- ^ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 801. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Dale L. Perry (May 19, 2011). Handbook of Inorganic Compounds (Second ed.). Taylor & Francis. p. 84. ISBN 978-1-4398-1461-1.
