 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 90% |
| Metabolism | Hepatic |
| Elimination half-life | 6,4-10,5 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.046 |
| Chemical and physical data | |
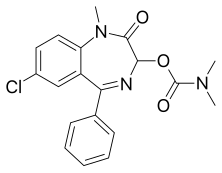
| Formula | C19H18ClN3O3 |
| Molar mass | 371.82 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Camazepam[2] is a benzodiazepine psychoactive drug, marketed under the brand names Albego, Limpidon and Paxor. It is the dimethyl carbamate ester of temazepam, a metabolite of diazepam.[3] While it possesses anxiolytic, anticonvulsant, skeletal muscle relaxant and hypnotic properties[4] it differs from other benzodiazepines in that its anxiolytic properties are particularly prominent but has comparatively limited anticonvulsant, hypnotic and skeletal muscle relaxant properties.
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ DE 2142181, "1,4 Benzodiazepine derivatives and process for their production", issued 23 December 1976, assigned to Siphar S A , Lugano (Schweiz).
- ^ Tammaro A, Picceo MT, Gemmellaro P, Bonaccorso O (1977). "Camazepam versus placebo. A double-blind clinical study on geriatric patients suffering from psychic complaints. Short Communication". Arzneimittel-Forschung. 27 (11): 2177–2178. PMID 23793.
- ^ Lu XL, Yang SK (April 1994). "Enantiomer resolution of camazepam and its derivatives and enantioselective metabolism of camazepam by human liver microsomes". Journal of Chromatography A. 666 (1–2): 249–257. doi:10.1016/0021-9673(94)80387-0. PMID 7911374.