 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.230.839 |
| Chemical and physical data | |
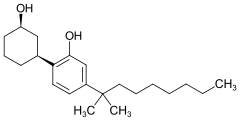
| Formula | C22H36O2 |
| Molar mass | 332.528 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cannabicyclohexanol (CCH, CP 47,497 dimethyloctyl homologue, (C8)-CP 47,497) is a cannabinoid receptor agonist drug, developed by Pfizer in 1979. On 19 January 2009, the University of Freiburg in Germany announced that an analog of CP 47,497 was the main active ingredient in the herbal incense product Spice, specifically the 1,1-dimethyloctyl homologue of CP 47,497, which is now known as cannabicyclohexanol.[2][3][4] The 1,1-dimethyloctyl homologue of CP 47,497 is in fact several times more potent than the parent compound,[5] which is somewhat unexpected as the 1,1-dimethylheptyl is the most potent substituent in classical cannabinoid compounds such as HU-210.[6]
- ^ Cook M (28 February 2008). "Synthetic marijuana illegal as of Tuesday". The San Diego Union-Tribune. San Diego. Retrieved 25 July 2015.
- ^ "Hauptwirkstoff von "Spice" identifiziert". University of Freiburg. 19 March 2009. Retrieved 25 July 2015.
- ^ Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreirós N (May 2009). "'Spice' and other herbal blends: harmless incense or cannabinoid designer drugs?". Journal of Mass Spectrometry. 44 (5): 832–837. Bibcode:2009JMSp...44..832A. doi:10.1002/jms.1558. PMID 19189348.
- ^ Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y (May 2010). "Chemical analysis of synthetic cannabinoids as designer drugs in herbal products". Forensic Science International. 198 (1–3): 31–38. doi:10.1016/j.forsciint.2010.01.004. PMID 20117892.
- ^ Compton DR, Johnson MR, Melvin LS, Martin BR (January 1992). "Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents". The Journal of Pharmacology and Experimental Therapeutics. 260 (1): 201–209. PMID 1309872.
- ^ Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, et al. (November 1991). "Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs". Pharmacology, Biochemistry, and Behavior. 40 (3): 471–478. doi:10.1016/0091-3057(91)90349-7. PMID 1666911. S2CID 19386120.