
| Part of a series on |
| Continuum mechanics |
|---|
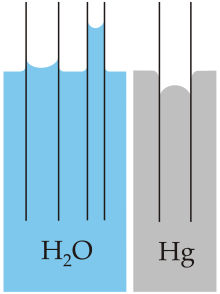
Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a liquid flowing in a narrow space without the assistance of external forces like gravity.
The effect can be seen in the drawing up of liquids between the hairs of a paint-brush, in a thin tube such as a straw, in porous materials such as paper and plaster, in some non-porous materials such as clay and liquefied carbon fiber, or in a biological cell.
It occurs because of intermolecular forces between the liquid and surrounding solid surfaces. If the diameter of the tube is sufficiently small, then the combination of surface tension (which is caused by cohesion within the liquid) and adhesive forces between the liquid and container wall act to propel the liquid.
