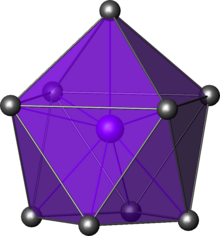
| Capped square antiprismatic molecular geometry | |
|---|---|
 | |
| Point group | C4v |
| Coordination number | 9 |
In chemistry, the capped square antiprismatic molecular geometry describes the shape of compounds where nine atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a gyroelongated square pyramid. The symmetry group of the resulting object is C4v
The gyroelongated square pyramid is a square pyramid with a square antiprism connected to the square base. In this respect, it can be seen as a "capped" square antiprism (a square antiprism with a pyramid erected on one of the square faces).
It is very similar to the tricapped trigonal prismatic molecular geometry, and there is some dispute over the specific geometry exhibited by certain molecules. Examples:
- [SiCo9(CO)21]2-, defined by the Co9 framework, which encapsulates the Si atom
- [Pb(phen)4(OClO3)]+, defined by the N8O framework, which encapsulates the Pb2+ ion
- [Ge9]4-, a zintl ion
- Th(troopolonate)4(H2O), defined by the O9 framework, which encapsulates the Th4+ ion
- ReH2−
9 is sometimes described as having a capped square antiprismatic geometry, although its geometry is most often described as tricapped trigonal prismatic. - [LaCl(H
2O)
7]4+
2, a lanthanum(III) complex with a La–La bond.[1]
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 917. ISBN 978-0-08-037941-8.