| |
| |
| Names | |
|---|---|
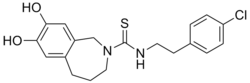
| Preferred IUPAC name
N-[2-(4-Chlorophenyl)ethyl]-7,8-dihydroxy-1,3,4,5-tetrahydro-2H-2-benzazepine-2-carbothioamide | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H21ClN2O2S | |
| Molar mass | 376.9 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Capsazepine is a synthetic antagonist of capsaicin.[1] It is used as a biochemical tool in the study of TRPV ion channels.
- ^ Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC (October 1992). "Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin". British Journal of Pharmacology. 107 (2): 544–52. doi:10.1111/j.1476-5381.1992.tb12781.x. PMC 1907893. PMID 1422598.