| |||
 Carbon tetraiodide crystals (left)
Solution in Et2O (right) | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Tetraiodomethane[1] | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 1733108 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.335 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CI4 | |||
| Molar mass | 519.629 g·mol−1 | ||
| Appearance | Dark violet crystals | ||
| Density | 4.32 g mL−1 | ||
| -136·10−6 cm3/mol | |||
| Structure | |||
| Tetragonal | |||
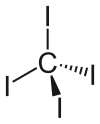
| Tetrahedral | |||
| 0 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
0.500 J K−1 g−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
384.0–400.4 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−794.4–−778.4 kJ mol−1 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
toxic | ||
| GHS labelling: | |||

| |||
| Warning | |||
| H315, H319, H335 | |||
| P261, P305+P351+P338 | |||
| Related compounds | |||
Other anions
|
Carbon tetrafluoride Carbon tetrachloride Carbon tetrabromide | ||
Other cations
|
Silicon tetraiodide Germanium tetraiodide Tin(IV) iodide | ||
Related alkanes
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Carbon tetraiodide is a tetrahalomethane with the molecular formula . Being bright red, it is a relatively rare example of a highly colored methane derivative. It is only 2.3% by weight carbon, although other methane derivatives are known with still less carbon.
- ^ "Tetraiodomethane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification and Related Records. Retrieved 29 February 2012.


