| |

| |
| Names | |
|---|---|
| IUPAC name
Carbonic acid[1]
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.133.015 |
| EC Number |
|
| 25554 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
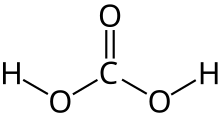
| H 2CO 3 | |
| Appearance | Colorless gas |
| Melting point | −53 °C (−63 °F; 220 K)[3] (sublimes) |
| Boiling point | 127 °C (261 °F; 400 K) (decomposes) |
| Reacts to form carbon dioxide and water | |
| Acidity (pKa) | |
| Conjugate base | Bicarbonate, carbonate |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Structure | |
| monoclinic | |
| p21/c, No. 14 | |
| - | |
a = 5.392 Å, b = 6.661 Å, c = 5.690 Å α = 90°, β = 92.66°, γ = 90°[4] (D
2CO 3 at 1.85 GPa, 298 K) | |
Lattice volume (V)
|
204.12 Å3 |
Formula units (Z)
|
4 formula per cell |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Carbonic acid is a chemical compound with the chemical formula H2CO3. The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature.[5][6] The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters.[4]
In biochemistry and physiology, the name "carbonic acid" is sometimes applied to aqueous solutions of carbon dioxide. These chemical species play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis.[7]
- ^ "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. P001–4. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b c Perrin, D. D., ed. (1982) [1969]. Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution. IUPAC Chemical Data (2nd ed.). Oxford: Pergamon (published 1984). "Carbonic Acid, H2CO3" entry. ISBN 0-08-029214-3. LCCN 82-16524.
- ^ W. Hage, K. R. Liedl; Liedl, E.; Hallbrucker, A; Mayer, E (1998). "Carbonic Acid in the Gas Phase and Its Astrophysical Relevance". Science. 279 (5355): 1332–5. Bibcode:1998Sci...279.1332H. doi:10.1126/science.279.5355.1332. PMID 9478889.
- ^ a b Benz, Sebastian; Chen, Da; Möller, Andreas; Hofmann, Michael; Schnieders, David; Dronskowski, Richard (September 2022). "The Crystal Structure of Carbonic Acid". Inorganics. 10 (9): 132. doi:10.3390/inorganics10090132. ISSN 2304-6740.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 310. ISBN 978-0-08-037941-8.
- ^ Loerting, Thomas; Tautermann, Christofer; Kroemer, Romano T.; Kohl, Ingrid; Hallbrucker, Andreas; Mayer, Erwin; Liedl, Klaus R.; Loerting, Thomas; Tautermann, Christofer; Kohl, Ingrid; Hallbrucker, Andreas; Erwin, Mayer; Liedl, Klaus R. (2000). "On the Surprising Kinetic Stability of Carbonic Acid (H2CO3)". Angewandte Chemie International Edition. 39 (5): 891–4. doi:10.1002/(SICI)1521-3773(20000303)39:5<891::AID-ANIE891>3.0.CO;2-E. PMID 10760883.
- ^ Acid-Base Physiology 2.1 – Acid-Base Balance by Kerry Brandis.
