 | |
| Clinical data | |
|---|---|
| Other names | 1-hexylcarbamoyl-5-fluorouracil, HCFU, N-hexylcarbamoyl-5-fluorouracil, Yamaful, NCGC00095165-01, Hexylcarbamoyl fluorouracil, 61422-45-5, UNII-HA82M3RAB2, CCRIS 2759, C11H16FN3O3, Uracil, 5-fluoro-1-hexylcarbamoyl-, BRN 0888898, HA82M3RAB2, 1(2H)-Pyrimidinecarboxamide, 5-fluoro-N-hexyl-3,4, |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.216.315 |
| Chemical and physical data | |
| Formula | C11H16FN3O3 |
| Molar mass | 257.265 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
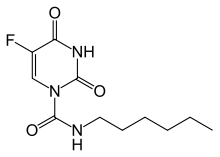
Carmofur (INN) or HCFU (1-hexylcarbamoyl-5-fluorouracil) is a pyrimidine analogue used as an antineoplastic agent. It is a derivative of fluorouracil, being a lipophilic-masked analog of 5-FU that can be administered orally.[1]
- ^ Shelton J, Lu X, Hollenbaugh JA, Cho JH, Amblard F, Schinazi RF (December 2016). "Metabolism, Biochemical Actions, and Chemical Synthesis of Anticancer Nucleosides, Nucleotides, and Base Analogs". Chemical Reviews. 116 (23): 14379–14455. doi:10.1021/acs.chemrev.6b00209. PMC 7717319. PMID 27960273.