 | |
| Clinical data | |
|---|---|
| Trade names | Biocef, Ceclor, Medacef, Distaclor, Keflor, Raniclor |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682729 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Well absorbed, independent of food intake |
| Metabolism | 15% to 40% |
| Elimination half-life | 0.6 to 0.9 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.053.536 |
| Chemical and physical data | |
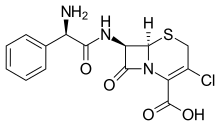
| Formula | C15H14ClN3O4S |
| Molar mass | 367.80 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cefaclor, sold under the trade name Ceclor among others, is a second-generation cephalosporin antibiotic used to treat certain bacterial infections such as pneumonia and infections of the ear, lung, skin, throat, and urinary tract. It is also available from other manufacturers as a generic.[1]
It was patented in 1973 and approved for medical use in 1979.[2]
- ^ "Drugs@FDA: FDA-Approved Drugs".
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 493. ISBN 9783527607495.