 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.148.965 |
| Chemical and physical data | |
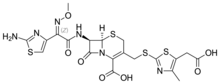
| Formula | C20H20N6O7S4 |
| Molar mass | 584.66 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cefodizime is a 3rd generation cephalosporin antibiotic with broad spectrum activity against aerobic gram positive and gram negative bacteria. Clinically, it has been shown to be effective against upper and lower respiratory tract infections, urinary tract infections, and gonorrhea. Cefodizime is a bactericidal antibiotic that targets penicillin-binding proteins (PBPs) 1A/B, 2, and 3 resulting in the eventual death of the bacterial cell. In vivo experimental models of infection showed that bacterial clearance by this drug is at least as effective compared with other 3rd generation cephalosporins. It has similar adverse effect profile to other 3rd generation cephalosporins as well, mainly being limited to gastrointestinal or dermatological side effects.[1]
It is not currently approved by the FDA for use in the United States.