 | |
| Clinical data | |
|---|---|
| Trade names | Xcopri, Ontozry |
| Other names | YKP3089 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620021 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ≥88% |
| Protein binding | 60% |
| Metabolism | Mainly glucuronidation via UGT2B7 |
| Elimination half-life | 50–60 hours |
| Excretion | Mainly via urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
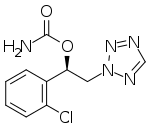
| Formula | C10H10ClN5O2 |
| Molar mass | 267.67 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Cenobamate, sold under the brand names Xcopri (US) and Ontozry (EU), is a medication used for the treatment of partial-onset seizures, a kind of epilepsy, in adults.[3][7][8] It is taken by mouth.[3]
Cenobamate was approved for medical use in the United States in November 2019,[3][7][8][9] and placed in Schedule V of the Controlled Substances Act in March 2020.[10] Cenobamate was approved for medical use in the European Union in March 2021,[5] approved for use in the UK in December 2021,[11] and approved for use in Canada in June 2023.[12]
- ^ "Details for: Xcopri". Health Canada. 20 November 2023. Archived from the original on 3 March 2024. Retrieved 3 March 2024.
- ^ "Notice: Multiple additions to the Prescription Drug List (PDL) [2023-08-30]". Health Canada. 26 October 2023. Archived from the original on 3 January 2024. Retrieved 3 January 2024.
- ^ a b c d "Xcopri Titration Pack- cenobamate kit Xcopri- cenobamate tablet, film coated Xcopri Maintenance Pack- cenobamate kit". DailyMed. Archived from the original on 11 August 2020. Retrieved 1 February 2021.
- ^ "Schedules of Controlled Substances: Placement of Cenobamate in Schedule V". Federal Register. 10 March 2020. Archived from the original on 3 April 2021. Retrieved 10 March 2020.
- ^ a b "Ontozry EPAR". European Medicines Agency (EMA). 25 January 2021. Archived from the original on 4 June 2021. Retrieved 4 June 2021. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "Ontozry Product information". Union Register of medicinal products. Archived from the original on 2 August 2021. Retrieved 3 March 2023.
- ^ a b "FDA approves new treatment for adults with partial-onset seizures". U.S. Food and Drug Administration (FDA) (Press release). 21 November 2019. Archived from the original on 22 November 2019. Retrieved 21 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Drug Trials Snapshots: Xcopri". U.S. Food and Drug Administration (FDA). 3 December 2019. Archived from the original on 19 December 2019. Retrieved 18 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Drug Approval Package: Xcopri". U.S. Food and Drug Administration (FDA). 10 December 2019. Archived from the original on 19 December 2019. Retrieved 18 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "2020 - Placement of Cenobamate in Schedule V". DEA Diversion Control Division. 10 March 2020. Archived from the original on 19 April 2020. Retrieved 11 March 2020.
- ^ "Information for the public - Technology appraisal guidance [TA753]". NICE. 15 December 2021. Retrieved 15 August 2024.
- ^ Mireku A (30 June 2023). "Health Canada approves Endo's anti-seizure pills". Pharmaceutical Technology. Retrieved 6 March 2024.