
| Part of a series on |
| Continuum mechanics |
|---|
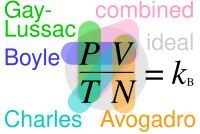
Charles's law (also known as the law of volumes) is an experimental gas law that describes how gases tend to expand when heated. A modern statement of Charles's law is:
When the pressure on a sample of a dry gas is held constant, the Kelvin temperature and the volume will be in direct proportion.[1]
This relationship of direct proportion can be written as:
So this means:
where:
- V is the volume of the gas,
- T is the temperature of the gas (measured in kelvins), and
- k is a constant for a particular pressure and amount of gas.
This law describes how a gas expands as the temperature increases; conversely, a decrease in temperature will lead to a decrease in volume. For comparing the same substance under two different sets of conditions, the law can be written as:
The equation shows that, as absolute temperature increases, the volume of the gas also increases in proportion.
- ^ Fullick, P. (1994), Physics, Heinemann, pp. 141–42, ISBN 978-0-435-57078-1.



