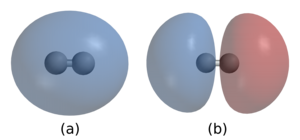
2. In (a) the two nuclei are surrounded by a cloud of two electrons in the bonding orbital that holds the molecule together. (b) shows hydrogen's antibonding orbital, which is higher in energy and is normally not occupied by any electrons.
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds, or some combination of these effects. Chemical bonds are described as having different strengths: there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force, and hydrogen bonding.
Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. Electrons shared between two nuclei will be attracted to both of them. "Constructive quantum mechanical wavefunction interference"[1] stabilizes the paired nuclei (see Theories of chemical bonding). Bonded nuclei maintain an optimal distance (the bond distance) balancing attractive and repulsive effects explained quantitatively by quantum theory.[2][3]
The atoms in molecules, crystals, metals and other forms of matter are held together by chemical bonds, which determine the structure and properties of matter.
All bonds can be described by quantum theory, but, in practice, simplified rules and other theories allow chemists to predict the strength, directionality, and polarity of bonds.[4] The octet rule and VSEPR theory are examples. More sophisticated theories are valence bond theory, which includes orbital hybridization[5] and resonance,[6] and molecular orbital theory[7] which includes the linear combination of atomic orbitals and ligand field theory. Electrostatics are used to describe bond polarities and the effects they have on chemical substances.
- ^ Levine, Daniel S.; Head-Gordon, Martin (2020-09-29). "Clarifying the quantum mechanical origin of the covalent chemical bond". Nature Communications. 11 (1). Springer Science and Business Media LLC: 4893. doi:10.1038/s41467-020-18670-8. ISSN 2041-1723. PMC 7524788. PMID 32994392. S2CID 222157102.
- ^ Pauling, L. (1931), "The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules", Journal of the American Chemical Society, 53 (4): 1367–1400, doi:10.1021/ja01355a027
- ^ Hund, F. (1928). "Zur Deutung der Molekelspektren. IV". Zeitschrift für Physik (in German). 51 (11–12): 759–795. Bibcode:1928ZPhy...51..759H. doi:10.1007/BF01400239. ISSN 1434-6001. S2CID 121366097.
- ^ Frenking, Gernot; Krapp, Andreas (2007-01-15). "Unicorns in the world of chemical bonding models". Journal of Computational Chemistry. 28 (1): 15–24. doi:10.1002/jcc.20543. PMID 17109434. S2CID 7504671.
- ^ Jensen, Frank (1999). Introduction to Computational Chemistry. John Wiley and Sons. ISBN 978-0-471-98425-2.
- ^ Pauling, Linus (1960). "The Concept of Resonance". The Nature of the Chemical Bond – An Introduction to Modern Structural Chemistry (3rd ed.). Cornell University Press. pp. 10–13. ISBN 978-0801403330.
- ^ Gillespie, R.J. (2004), "Teaching molecular geometry with the VSEPR model", Journal of Chemical Education, 81 (3): 298–304, Bibcode:2004JChEd..81..298G, doi:10.1021/ed081p298